Standard Operating Procedure (SOP) for Cleaning Validation (CV) in the pharmaceutical drug manufacturing plants. Cleaning Validation (CV) is the documented evidence that an approved cleaning procedure is consistent in reducing product residue and removal of cleaning agents (if any), bioburden, flavor (if any), color (if any) from equipment and accessories within the acceptance level.
Procedure for Cleaning Validation (CV)
1.0 PURPOSE:
-
- The purpose of this procedure is to prove that the equipment cleaning procedure can consistently clean the previous product, the cleaning agent (if any), and microbial residues to an acceptable level to prevent possible contamination and cross-contamination.
-
- To decide the worst-case product for performing cleaning validation (CV) for common equipment.
2.0 SCOPE:
-
- This SOP is applicable for validating cleaning procedures followed for process equipment and accessories used in manufacturing pharmaceutical products.
3.0 RESPONSIBILITY – CLEANING VALIDATION (CV):
-
- Production: Cleaning of equipment and accessories as per respective SOP.
-
- Utility: Equipment surface area calculation.
-
- Quality Assurance: Sampling and data compilation.
-
- Quality Control: Microbial sampling & Analysis of samples.
-
- Head of Production, QC, and QA: Review and approval of the cleaning validation (CV) protocol and report.
4.0 DEFINITION (S):
-
-
Cleaning Validation (CV):
-
-
- It is documented evidence that an approved cleaning procedure is consistent in reducing product residue and removal of cleaning agents (if any), bioburden, flavor (if any), color (if any) from equipment and accessories within the acceptance level.
-
-
Worst case:
-
-
- A product or set of conditions encompassing the upper and lower processing limits for operating parameters and circumstances with SOP which pose the greatest chance of product or process failure when compared to ideal conditions. Such conditions do not necessarily include product or process failure.
-
-
Worst case product:
-
-
- The product selected from a group of products that represents the greatest risk of carry-over contamination to other products made in the same equipment by virtue of its poor solubility, potency, and toxicity, or a combination of these factors.
-
-
Bracketing:
-
-
- Grouping of products manufactured in identical equipment chains from which the worst-case product will be selected based on batch size, solubility, daily doses, and therapeutic dose.
-
-
Acceptance criteria:
-
-
- To demonstrate during validation that the cleaning procedure, routinely employed for a piece of equipment, limits potential carryover to an acceptable level.
-
-
Revalidation:
-
-
- The repeat of initial validation either after changes/introduction to equipment, new product or periodically to provide assurance that the changes are done, do not affect the cleaning effectiveness.
5.0 PROCEDURE – CLEANING VALIDATION (CV)
- Ensure that the prerequisites for cleaning validation (CV) as listed below are available:
-
- Approved Cleaning Validation (CV) Protocol. (Click here for Protocol)
-
- Validated analytical Method for estimation of the previous product (API) in the rinse and swab sample.
Note: The efficiency of the recovery of API by the analytical method should be assessed.
-
- The product matrix shall be available w.r.t. details of solubility, dosage, the toxicity of active ingredient(s), and batch sizes of products manufactured in the existing facility.
-
- The validated analytical method for estimation of cleaning agent (if any) in the rinse/swab.
-
- Approved product contact surface area of equipment involved in the manufacturing of pharmaceuticals product.
-
- The basic information or contact surface area of equipment pieces may be taken from manufacturer documents also.
-
- Approved sampling plan (for both chemical and microbiological sampling) taking the sample from complexity and design of equipment into consideration.
-
- Approved swab locations for the equipment involved in the manufacturing of pharmaceuticals product.
-
- Product Vs. Equipment matrix with contact surface area.
-
- Approved equipment cleaning SOPs involved in manufacturing.
-
- The time frame for storage of uncleaned equipment for cleaning shall be established (unclean equipment may be stored up to 72 hours).
-
Cleaning of Equipment – Cleaning Validation (CV):
-
- Clean the equipment as per respective approved cleaning SOP available ensuring the following:
-
-
- The number of cleaning steps and/or cycles shall be performed as per respective equipment cleaning SOPs.
-
-
-
- The volume of purified water / WFI shall be used for the final rinsing of equipment/equipment parts as per individual SOPs or respective annexures of cleaning validation (CV) protocol.
-
-
-
- Purified water shall be used as a final rinse for equipment, to be used in the production of non-sterile products.
-
-
-
- WFI shall be used as the final rinse for equipment to be used in the production of sterile products.
-
-
Collection of samples for chemical & Microbial analysis – Cleaning Validation (CV):
-
- Collect the swab/rinse sample of each piece of equipment involved for manufacturing after final cleaning as per the approved sampling plan.
-
- The final rinse sample shall be collected in a way that the sample representative of the entire rinse volume.
-
- Withdraw about 100 ml rinse volume for active ingredient from the final rinse for measurement of the active ingredient and collect the individual swab from the equipment part to estimate the cleaning agent used (if any).
-
- Swab individually various parts of the equipment after cleaning and final rinsing of parts as detailed in the sampling plan.
-
- The selection of swabbing sites shall be hard to clean sites with justification.
-
- Refer individual sampling plan of different equipment’s parts to be swabbed (as per cleaning validation (CV) protocol).
-
- Microbial swab samples shall be collected prior to a chemical swab sample.
-
- The Selection of solvent for a swab, if other than water shall be based on the solubility of the active ingredient.
-
- The cleaning of the equipment (CIP and COP) shall be done in all three validation runs by different operators to verify the ruggedness of the cleaning procedure.
-
Labeling and storage of cleaning validation (CV) sample :
-
- Label each swab and rinse the sample appropriately as per Annexure no. III (specimen label).
-
- The rinse sample shall be stored in an amber color bottle and swab sample inappropriately covered glass test tube with proper labeling so as to prevent contamination or alteration during storage.
-
- Microbial swab samples shall be store in the sterile well-closed test tubes with proper labeling.
-
Visual evaluation of cleaning– Cleaning Validation (CV):
- Visually inspect the final rinse of equipment/each part of the equipment to ensure that it is clean, clear, and colorless.
-
- Use a torch, mirror, etc for verification of cleanliness wherever direct access of area is not possible.
-
- Production/QA personal assessing visual cleanliness shall be trained for observing and identifying drug substances at low-level concentration.
-
- Training shall be provided by subjecting officers to review and identify the drug substance residue at a lower level which is generated by spotting solutions of lower concentration (at LOD level) on all MOC involved in equipment cleaning, performed during recovery studies conducted by the laboratory for method validation of the analytical method.
-
Odor Verification – Cleaning Validation (CV):
-
- In formulation where flavors/pungent are used or where the materials are used has itself typical odor,
-
- Ensure that final rinse/sample rinse and equipment are free from the characteristic odor of the previous product shall be verified by the smelling of cleaned equipment part.
-
- Measure the content of API in rinse/swab as per the approved analytical method in QC.
-
- Confirm the presence of the cleaning agent used (if any), in the final rinse, swab/rinse sample within an acceptable level.
-
- For solvents other than water and volatile organic solvents, when used for cleaning of equipment, residues of solvents shall be checked in addition to API and cleaning agent.
-
- Based on the analysis, calculate the amount of residue present in each rinse/swab, and on the basis of rinse/swab result and measure probable contamination in the next product, calculate the amount of residue present in each equipment involved.
-
Microbiological aspects of Cleaning Validation (CV):
-
- Bioburden study of equipment shall be performed, after cleaning/sanitization to ensure microbiological cleanliness.
-
- Check visually no stagnant water shall be allowed to remain in the equipment subsequent to cleaning operation.
-
- Equipment should be dry before storage by an appropriate method of drying as per SOP or allow all the water to drain from the equipment and its parts.
-
Establishment of Acceptance Criteria – Cleaning Validation (CV):
- The limits for Acceptance Criteria shall be decided based on factors such as batch size, therapeutic dosage, solubility, toxicology, and equipment surface area.
-
- The limit for cleaning validation’s acceptance criteria shall be established following four criteria:
-
-
Criteria 1: Dose Criteria : (Cleaning Validation -CV)
-
-
- NMT 0.1 % of the normal therapeutic dose of any product will appear in the maximum daily dose of the next product. OR
-
- Not more than 0.001 part of the minimum dose of the previous preceding product should appear in the maximum daily dose of the next considered product.
-
- Dose criteria shall be calculated by the following formula.
MAR (mg/swab) = STD X 0.001 X Mini B. size (mg) X 25 cm2
MDD (mg) X TSA
Where:
STD: Smallest therapeutic dose in mg of product
0.001: Safety factor
25 cm2: Swab surface area
MDD: Maximum daily dose in mg of product
TSA: Total equipment surface area
Mini B. size: Minimum batch size of the succeeding product
OR following formula also can be used:
I x K x M = ______mg of previous product/25 cm2
J L
Where,
I = 0.001 of the minimum dose of previous product in the equipment chain in mg
J = Maximum number of dosage units of next considered product taken/day in the equipment chain
L = Surface area of equipment common for both the products (previous & next product) in equipment chain
K = Minimum number of dosage units (Batch size) per batch of next considered product in equipment chain
M = 25 (for reporting results per 25 cm2)
-
-
Scientific rationales for the above statements are:
-
-
- Pharmaceuticals are often considered to be non-active at 0.1 part of their normally prescribed dosage (1/10th).
-
- The second is a safety factor of 10 (i.e. 1/10th of the above step).
-
- The third factor of 10 is included to make the cleaning procedure robust and to overcome variations due to personnel and sampling methodology (i.e. 1/10th of the above step).
-
- If similar equipment is used repeatedly in a chain, surface area to be considered for each time of usage during the calculation of the total surface area.
-
-
Criteria 2: 10 ppm Criteria
-
-
- Not more than 10ppm of the previous products should appear in a subsequently produced succeeding product.
-
- The 10 ppm criteria shall be calculated as per the following formula.
mg/swab = R X S X U = ______mg of previous product/25 cm2
T
Where,
R = 10 mg of active ingredients in previous product/kg of next considered product
S = Batch size in kg of next considered product.
T = Surface area of equipment common for both the products (previous and considered next product)
U = swab surface 25 cm2 (for reporting results per 25 cm2)
-
- Scientific rationales for the above statements are:
-
- It is based on regulations for the food industry which provides for a maximum permissible limit of certain levels of hazardous substances considered as acceptable in products that enter the human food chain.
-
Criteria No. 3: Toxic dose criteria (Cleaning Validation – CV)
-
- Wherever therapeutic dose is not known then toxicity criteria shall be applicable for cleaning validation study.
-
- The toxicity is expressed as LD50 can be used to calculate the maximum allowable limit.
-
- The following methodology can be used.
ADI = NOEL X AAW X SF
Where:
NOEL = LD50 X empirical Factor
NOE: No Observed Effect Level
LD50: Lethal dose for 50% of the animal population in the study
Empirical factor: Derived from animal model
ADI: Acceptable Daily Intake
AAW: Average Adult Weight (i.e. 70 Kg)
SF: Safety factor, to be taken as 1/1000
-
- This equation can be applied to a pharmaceutical cleaning validation study for the purpose of calculating a limit.
-
- The result would be as follows…
MAR = ADI X B and mg / swab = MAR x 25 cm2
R Total equipment surface area
Where:
MAR: Maximum Allowable Residue
B: The smallest batch size of any subsequent product.
R: Largest daily dose of any product made in the same equipment
Toxicity Table-1 (Cleaning Validation)
| Probable oral lethal dose for humans (mg/kg) | Included descriptive terms | Group |
| >150005000 – 15000 | Practically non toxicSlightly toxic | 1 |
| 500 – 5000 | Moderately toxic | 2 |
| 50-500 | Very toxic | 3 |
| 5 – 50 | Extremely toxic | 4 |
| >5 | Super toxic | 5 |
-
Criteria No. 4: Visually Clean Criteria (Cleaning Validation)
-
- No quantity of residue should be visible with naked on the equipment after the cleaning procedure is performed.
-
- The scientific rationale for the above statement is:
-
- Most of the product is visible to a normal human eye at approximately 100 µg per 25 cm2 of surface area.
-
- Generally, below this level, the residues are not visible to the human naked eye.
-
- The most stringent value from the above four criteria shall be considered as acceptance criteria for cleaning validation including visual criteria.
-
Selection of the worst-case products for performing cleaning validation
-
-
Worst case product based on solubility
-
-
- Active ingredients having the least solubility (Refer Table-2) in their cleaning solvent are most difficult to clean and the possibility of carryover contamination of that ingredient into the next product.
-
- The product having the worst solubility profile in their cleaning solvent/media shall be selected as the worst case product in the criterion.
-
- The data of solubility of API in solvents shall be taken from “Martindale/Merck index/Pharmacopoeias/Internet etc.
-
- In the case where the solubility profile of two or more products is identical, the product having the highest strength shall be selected as the worst case in this criterion.
-
- Whenever a worst-case product has two or more actives with different solvents used for cleaning, for both actives, study the solubility of each of the actives in both the solvents and shall be taken into consideration for validation activity of poor solubility in solvents and the highest strength.
Interpretation of solubility: Table-2
| Solubility | Approximate quantities of solvent by volume for 1 part of solute by weight. |
| Very soluble | Less than 1 part. |
| Freely soluble | From 1 to 10 parts |
| Soluble | From 10 to 30parts |
| Sparingly soluble | From 30 to 100 parts |
| Slightly Soluble | From 100 to 1000 parts |
| Very slightly soluble | From 1000 to 10000 parts |
| Insoluble Or Practically insoluble | More than 10000 parts |
-
-
Worst case product on the basis of least therapeutic dose (potency)
-
-
- The product having maximum potency possess maximum health hazard if contaminated in other product.
-
- The product having the least therapeutic dose is considered to be most potent and use for the establishment of acceptance criteria.
-
- Perform the cleaning validation studies with the selected worst-case product on the identified equipment chain for three consecutive runs.
-
Procedure for sampling during Cleaning Validation:
-
-
Procedure for rinse sampling
-
-
- Clean the equipment as per respective equipment cleaning SOP’S
-
- Collect the rinse sample as specified in the individual sampling plan.
-
- Rinse sampling shall primarily be used in cases where the surfaces are difficult to clean/reach.
-
- Following the type of rinse, sampling may be possible
-
- A] Holding type: This method shall be followed, by means of equipment design; it is possible to retain the rinse volume.
-
- B] Non-approachable piping: This method shall be used where volume retention is not possible by the open virtue of equipment design, eg, open-ended pipelines, dismantled parts, etc.
-
- Rinse sample can be collected using any type mentioned above, depending upon the requirement
-
-
Procedure for swab sampling:
-
-
- After completion of the cleaning of equipment swab samples shall be taken along with the required number of appropriately labeled test tubes with screw cap, swab stick following area’s gowning procedure.
-
- Swab sampling for bioburden study shall be carried out by QC person as per respective SOP.
-
- Do not touch the swab wick with hand.
-
- Depending on the product, moistens the swab (Texwipe®) with appropriate solvent/purified water, use hand gloves, nose mask, and snood wherever required.
-
- Immediately after wetting the swab wick, swab the specified equipment surfaces as per the sampling plan.
-
- The surface area to be swabbed is 25 sq. cm using the 5 x 5 cm template.
-
- If the surface area is less than 25 sq. cm, complete surface area should be swabbed.
-
- The swab sample will be taken after the final rinse of the equipment surface, which is hard to clean. Swab locations shall be determined based upon logic and practical approach.
-
- Equipment geometry also shall be considered and the same shall be justified in the respective sampling plans.
-
- In case of surfaces where 25 cm2 measurements for swab sampling are not possible like pipes, cavities groves mesh, etc.
-
-
Following approach should be adopted:
-
-
- A] Swab the surface such that it will cover the maximum possible accessible area.
-
- For example, in pipes, do the swabbing in a circular motion from the outer edge to the inner surface in the clockwise direction and return the swabbing in a similar procedure. i.e. from inside to outside in an anti-clockwise direction. For calculation retain the area of 25 cm2.
-
- B] If the swab surface area is non-uniform, an equivalent area of 25 cm2 shall be selected for the collection of the swab.
-
- Stainless steel/ Teflon/ Silicon/ PVC etc templates shall be used for determining the surface area of the swab, or eyeball method be practiced and validated for each sampling personals
-
- Swabbing is done in painting motion across the surface, first applying the swab in a vertical motion, and then applying the swab (after rotating it 90°) in a horizontal motion with the reverse surface of the swab.
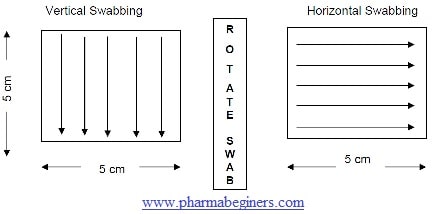
-
- Transfer the swab using gloved worn hand into the test tube and analyze it as per the validated analytical method.
Note: Ensure that micro swab samples are collected prior to the chemical swab sample.
-
- Swabbing and analysis for micro swab sample analysis shall be carried out as per respective SOP.
-
- Swab and rinse sampling shall be done for routine cleaning validation.
-
- However, swab sampling shall be considered as the primary criteria for cleaning validation.
-
- The cleaning validation samples analysis shall be carried out on HPLC and UV both (If the analysis is possible on both and analytical method has been carried out successfully on both) for rinse as well as swab samples to verify the result of samples and comparison between them for equipment train.
-
- If the results of rinse sampling considering the final rinse volume and the limit of detection for rinse samples are observed to be more from the acceptance criteria,
-
-
Then only swab sampling shall be done and the cleaning validation exercise shall be concluded based on the result of the swab sampling only.
-
-
- If the first swab sample result is out of limit re-clean the same equipment with a suitable solvent or by water and the same shall be addressed during cleaning validation and.
-
- Swab sampling site shall not be repeated and swabbing shall not be done from the same location of equipment where the swab sample is already collected before.
-
- The swab sample shall be collected adjacent to the defined sampling location where the sample is already collected.
-
- The cleaning validation activity shall be planned further for three consecutive batches with the consideration of a validated cleaning procedure as applied to previous batches.
-
- The same procedure shall be applicable for that particular product during routine cleaning activities after the successful completion of cleaning validation.
-
- While carrying out hold time study for dirty and clean equipment, swab samples should be collected from the surface of equipment as per swab sampling location.
-
- Swab sampling site shall not be repeated and re-swabbing shall not be done from the same location of equipment where the swab sample is already collected before.
-
- The swab sample shall be collected adjacent to the defined sampling location where the sample is already collected.
-
Procedure for analysis and evaluation of results for rinse & swab sample
-
- Analyze the swab and rinse sample in the laboratory using a validated analytical method.
-
- Apply recovery factor (obtained from validation study) for calculating the content, if the same is found less than 100%. If recovery is obtained more than 100%, do not apply factor for calculation.
Results = Observed value X 100
Recovery factor
-
- For example:
-
- If the recovery of the method is 80% and result obtained is 2.0 PPM, then apply recovery factor as follows:
Content (PPM) = (2.0 X 100)/80 = 2.5 ppm
-
- Limit of detection and limit of quantification shall be reported by QC after the establishment of the analytical method in Annexure-I after each introduction of the new product.
-
-
Acceptance criteria for residual solvents:
-
-
- If any solvents are used for cleaning of equipment, results obtained for residual solvent should be less than 1/10th of the ICH specified limit. The same shall be reported in the respective cleaning validation report.
-
-
Cleaning agents: Cleaning agents used should be easily removable. The cleaning agent should be absent (at LOD level) in the final rinse.
-
-
- Quality control laboratory shall provide the results of samples analyzed along with the limit of detection (for rinse as well as swab technique) of the analytical method used to analyze cleaning validation samples.
-
- The quality assurance shall verify the compliance of all the results obtained for the final rinse and swabs, which should be less than the acceptance criteria established.
-
- If results reported for rinse/swab samples by the laboratory are below the detection limit (Below LOD), the detection limit shall be considered as residue and evaluated against acceptance criteria for compliance.
-
- The detection limit of swab/rinse should be less than the acceptance criteria.
-
- Any failure to meet the acceptance criteria shall be jointly investigated by production, QC, and QA to determine the cause and appropriate actions (for example reanalysis, re-cleaning, cleaning by another method, etc.) shall be taken as necessary.
Note: If the cleaning method is being changed after the failure of the result then again three consecutive cleaning runs should be validated using a changed cleaning method.
-
- In the case of the theoretical acceptance criteria are found less than the LOD of the analytical method, the following actions to be initiated:
-
-
- An analytical method to be optimized to achieve the lower limit of detection by slight modification such as increasing injection volume in case of chromatographic method like HPLC/GC etc or increasing cell length in case of UV methods from 1 cm to 4/5 cm path length cell.
-
-
-
- If the above modification does not provide a limit of detection lower than the acceptance criteria established, a new method to be developed, which can achieve the required lower detection concentration. In case of modification, the method should be revalidated.
-
-
-
- More surface area can be considered for swabbing (for example 100 sq. cm).
-
-
-
- In the case of rinse sampling, the volume of sample rinse can be decreased resulting in an increase in the residue concentration and hence can be easily detected.
-
-
-
- If the swabbing area is modified, acceptance criteria also need to be corrected and recalculated with the revised area.
-
-
-
- When no methods can compliance the required acceptance criteria then LOD may be taken into consideration for acceptance criteria and calculation purposes.
-
-
-
- If the cleaning procedure consistently reduces the contaminants to a level within the limit of acceptance criteria, then the procedure being followed for cleaning can be regarded as validated.
-
-
Calculation of the amount of residue present in rinse and swab
-
-
Rinse method for Cleaning Validation:
-
-
- Content/residue of previous product in the rinse is reported in ppm by QC.
-
- It should be converted into milligram by multiplying the QC result with the quantity of rinse in Kg (i.e. quantity of water for final rinsing in Kg).
-
- The resultant value is the residue of previous product/s in milligram from entire equipment/part of equipment cleaned.
-
- For possible contamination per 25 sq. cm in the next considered product, the resultant value is multiplied by 25 and divided by surface area of the equipment/part of the equipment cleaned.
Example:
The residue of the previous product (from QC) = 2.58 ppm
Quantity of final rinse for equipment/equipment parts = 5 kg
Content/residue of previous product in mg = 2.58×5 = 12.9mg
Possible contamination in next considered product per 25 sq. cm = 12.9 x 25
9500
= 0.0334 mg/25 cm2
Where 9500 cm2 is the surface area of the equipment/part of the equipment.
-
Swab method – Cleaning Validation
- Content/residue of previous product is reported in mg per 25 cm2 by QC.
-
- If the result reported in ppm then calculate as follows:
-
- The observed ppm value is to be converted into mg by dividing the ppm value with 1000.
-
- This is the possible contamination in the next considered product in 25 cm2.
Example:
Content residue in ppm from result of analysis = 9.12 ppm
Possible contamination in next considered product = 9.12/1000 mg x dilution factor
= 0.091 mg/25 cm2 (with consideration of 10 ml dilution factor)
- If swabbing area is less than 25 cm2 then QC have to report the result in mg/swab.
Example:
Content/residue in ppm from results of analysis = 9.12 ppm
If swab is collected from 15 cm2 then = (9.12 x 25)/15
= 15.2 ppm/25 cm2
Contamination in next considered product = 15.2/1000 mg x dilution factor
= 0.152 mg/25 cm2(with consideration of 10 ml dilution factor).
Note: In the case of thermolabile API, for cleaning validation, only the swab method should be followed, as for the rinse method, the rinse will be evaporated at high temperature and this can cause degradation of temperature-sensitive API and will affect the subsequent analytical results.
-
- In the case of new product introduction in the facility, evaluation/assessment shall be done as per Annexure-I against existing worst-case products based on assessment report shall be decided that the product becomes worst-case or not.
-
-
If the product is worst-case then cleaning validation must be carried out with the same equipment chain.
-
-
- One batch of every new product shall be taken as a cleaning verification study with swab sampling only and shall be reported as per the annexure of the cleaning verification protocol.
-
- Whenever introduction, elimination or modification of any equipment evaluation /assessment shall be done as per annexure no. II, or
-
- Change in the next product considered for calculation, the surface area calculation shall revise and if the acceptance criteria emerged from the new calculation more stringent than the existing limit,
-
- The existing cleaning validation shall be compared with the new limits and if required, revalidation to be done for all worst-case products.
-
- If the equipment which has the minimum surface area is removed from the facility and the same equipment with maximum surface area and same cleaning procedure still is in the area then not required for validation or not required to revise the surface area of equipment due to worst-case study,
-
- But if the equipment with maximum surface area is removed then only total surface area shall be revised and thereafter the acceptance criteria may be revised (lower than existing) but revalidation is not required.
-
- If the equipment which has the minimum surface area than existing is introduced in the facility and the same equipment with maximum surface area and same cleaning procedure (validation) still is in the area then not required for validation or not required to revise the surface area of equipment in the chain due to worst-case study
-
- But if the equipment which has the maximum surface area than existing is introduced in the facility then the total surface area shall be revised accordingly and assessment shall be made for cleaning validation result for acceptance criteria and actually carry over to the next product during cleaning validation.
-
-
If the actual carryover is more than the new acceptance criteria, the cleaning validation study shall be planned on three consecutive batches.
- If one equipment chain has products which are common for another equipment chain, and if the surface area of the former is greater than the later,
-
-
- Then validation of the worst case of the former equipment chain will also justify the cleaning validation of the later, even the worst-case product of both the chains does not match.
-
- The statement can be justified as if worst-case products of the worst equipment chain (having maximum surface area) are validated successfully,
-
- Then the worst-case products of any other equipment chain (lower surface area) need not be validated separately.
-
- Provided the worst list of the worst chain includes products of other chains also and cleaning procedure is the same for equipment used in both chains.
-
- This because, the worst product is already considered in the worst chain, and is being validated.
-
For example:
-
- Consider chain 1 having products A, B, C, D, E, and F in its product list and among which products A and C are the worst cases.
-
- Similarly, chain 2 has products B, D, E, and F in its product list and among which products B and E are the worst cases.
-
- In the surface area chain, 1 is greater than chain 2 and if products A and C are validated in chain 1, then the cleaning procedure will stand validated in chain 2 also, even if products B and E are not validated in chain 2.
-
- This can be justified as although products B and E are not validated in chain 1, still, the same cleaning procedure is effective in cleaning products more worst than the above products (A and C).
-
- Note: If cleaning procedures are different, worst products in the equipment chain, the worst-case shall be validated.
-
-
Whenever the introduction & deletion of equipment and products following document shall be updated but not limited to:
-
-
-
- Equipment matrix
-
-
-
- Product matrix with their solubility profile
-
-
-
- MAR calculation sheet
-
-
-
- A regular validation review must be established to maintain the validated status of the cleaning procedure.
-
-
- Carry out re-validation in case of a change in equipment (if not identical and surface area is more than the existing and actual validated result is more than the new acceptance criteria), changes in established cleaning method, the introduction of the new worst-case product (May not be required if the assessment is satisfactory on the existing worst-case actual result and new worst-case acceptance criteria)
-
-
Periodic Cleaning Re-Validation:
-
-
- If no cleaning validation required or not done on the next worst-case within 03 years then revalidation shall be carried out on existing worst in the frequency of 03 years,
-
- whenever worst-case products to be planned for manufacturing.
6.0 ABBREVIATIONS – SOP FOR CLEANING VALIDATION
-
- ICH: International Conference on Harmonization
-
- LOD: Limit of detection
-
- LOQ: Limit of quantification
-
- HPLC: High-performance liquid chromatography
-
- UV: Ultraviolet
-
- MACO: Maximum allowable carry-over
7.0 REFERENCES – SOP FOR CLEANING VALIDATION
-
- WHO Technical Report Series 937, Fortieth report
-
- European Commission, Brussels, 30 Oct. 1999, E-3 D (99)
-
- APIC A sector group of CEFIC 2000
8.0 ANNEXURES
-
Annexure –I: Comparison of the worst-case product after the introduction of a new product (for both the present and next product of each criterion).
A) Comparison of product specification
| Sr. No. | Product description | Worst-case Specification | New Product Specification | |
| 1. | Name of product | |||
| 2. | Name of the active ingredient | |||
| 3. | Strength of product | |||
| 4. | Solubility profile of the active ingredient | |||
| 5. | Maximum daily dose | |||
| 6. | Batch size of the product | |||
| 7. | MAR value | |||
| 7.1 | 10 ppm Criteria | |||
| 7.2 | Dose criteria | |||
| 7.3 | Toxicity criteria | |||
B) New product comparison with the worst-case product
-
- The solubility of new product < Solubility of worst-case product. Yes ……… No…….
-
- MAR value calculation of new product< MAR worst-case product. Yes ……… No…….
-
- The maximum daily dose of new product > Maximum daily dose of worst-case product. Yes… No….
-
- Total surface area of the equipment ___________________
-
- Inference: ——————————————————
C) Calculation of MAR:
-
- 10 ppm Criteria:_____________________________
-
- Dose criteria:_______________________________
-
- Toxicity criteria: _____________________________
-
- Inference: ———————————————————————–
- Cleaning validation required for the newly added product/s. Yes ……… No…….
-
- If Yes then;
-
- Limit of mg/swab: ___________ and ___________ ppm/swab
D) Quality control review:
-
- LOD-Limit of detection of API on HPLC:
-
- LOD-Limit of detection of API on UV:
-
- LOQ-Limit of quantification of API on HPLC:
-
- LOQ-Limit of quantification of API on UV:
-
- LOD-Limit of detection for cleaning agent:
| Swab recovery (%) on following MOC | ||||||||
| Recover done by | SS | Silicon rubber | Nylon | Latex Rubber | Neoprene Rubber | Polycarbonate | Polypropylene | |
| HPLC | ||||||||
| UV | ||||||||
| Teflon | Acrylic | Glass | Brass | Gun Metal | FG260/SGI600/3 | OHNS | HCHC | S7 | Aluminum |
| Rinse recovery (%) on following MOC | |||||||||
| Recover done by | SS | Silicon rubber | Nylon | Latex Rubber | Neoprene Rubber | Poly carbonate | Poly propylene | Teflon | |
| HPLC | |||||||||
| UV | |||||||||
| Acrylic | Glass | Brass | Gun Metal | FG260/SGI600/3 | OHNS | HCHC | S7 | Aluminum |
- Swabbing solvent with concentration and ratio:______________
E) Acceptance Criteria Equipment wise:
-
- Total Equipment surface area in the equipment chain (a):_____________
-
- Lowest MAR value among all three criteria taken into consideration (b):___________
| Sr. No. | Equipment Name | Equipment surface area in cm2 (c) | Acceptable contamination in mg per equipment {(b/a)xc} |
-
Annexure-II: Comparison of worst-case equipment after the introduction/deletion of equipment.
A) Assessment of equipment addition/deletion
| Sr. No. | Description | Equipment details |
| 1. | Name of equipment | |
| 2. | Volumetric capacity | |
| 3. | Equipment ID no. | |
| 4. | Equipment added/deleted in/from area | |
| 5. | Equipment surface area |
B) Comparison with existing equipment chain
-
- Any other similar equipment in the facility. Yes________ No ________
-
- Equipment added/deleted in/from equipment chain no.: ___________
-
- Total surface area of the equipment in this chain ___________________
-
- Total surface area of equipments after introduction of new equipment in this chain_____________
-
- Inference: ————————————————
-
Annexure – III: Status label of swab/rinse sample
|


