Standard Operating Procedure (SOP) and Guideline for preparation of Equipment / System Qualification (URS, IQ, OQ, PQ, FAT, SAT, etc.) documents, execution of Qualification activities, Review and Compilation of data, Assessment and Interpretation of Qualification & validation activity results.
Equipment and System Qualification
1.0 Purpose :
To lay down the procedure for preparation of Qualification documents, execution of Qualification activities, Review and Compilation of Data, Assessment, and Interpretation of Qualification & validation activity results.
2.0 Scope :
The standard operating procedure applicable for the preparation of qualification and re-qualification documents for all equipment & system and execution of qualification activities performed.
3.0 Responsibility :
Officer and above of User, Engineering and QA Department shall be responsible for the preparation and execution of the equipment and system Qualification and Re-qualification activities
4.0 Accountability
Head- User, Engineering, and Quality Assurance shall be responsible for compliance.
5.0 Procedure – Equipment and System Qualification :
- Qualification Approach – Equipment and System :
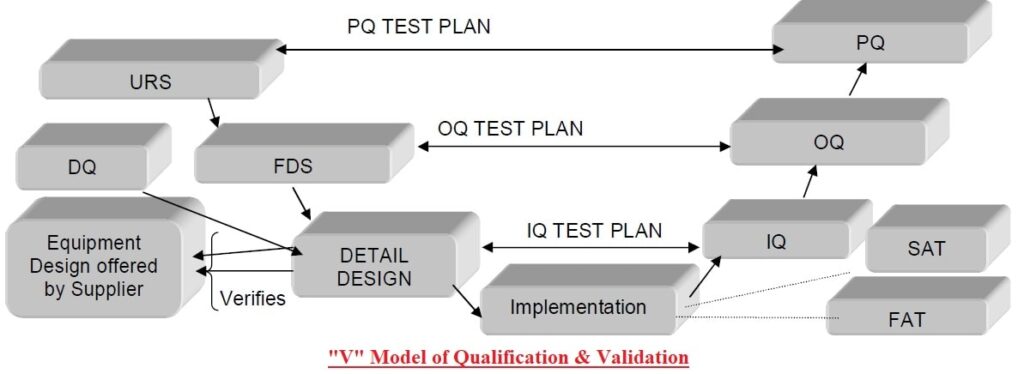
- The universal “V model” approach shall be followed for all the Qualification and Validation activities.
- The model is as follows:
-
- The left arm of the “V” deals with defining the requirement and design of the equipment.
-
- The right arm of the “V” ensures that for each item in the left arm, there is a corresponding activity that verifies the same in the equipment.
-
- These include:
-
-
- The installation is as per the design specification in the URS (this activity is called IQ).
-
-
-
- The Operation is as per the function and design specification in the URS (this activity is called OQ).
-
-
-
- The performance is as per the overall performance requirements specified in the URS (this activity is called PQ).
-
-
-
FAT & / or SAT may be done based on the risk assessment or criticality of the equipment; it is not mandatory and hence, is shown with dotted lines.
-
-
- Qualification activity shall consider stages from the initial development of the user requirement specification through to the end of the use of the equipment, facility, utility, or system.
-
- The introduction of any new equipment or system shall follow the quality risk management approach for qualification activity.
-
- The impact of equipment or system shall be assessed during risk assessment to determine that equipment or system that requires qualification and that equipment, which do not have this requirement.
-
- Based on the impact assessment, the equipment or system shall be categorized as “Direct Impact”, “Indirect Impact” and “No Impact”.
-
- Equipment or system shall be defined as Direct Impact on Qualification if it meets any of the following criteria.
-
-
- It has direct contact with the product which is impacting product quality attributes. E.g. Air quality, MOC, etc.
-
-
-
- To provide or produce an in-process material or an ingredient /excipient or solvent, which forms a part of final product formulation e.g. purified water
-
-
-
- It is used in cleaning and sterilization of critical equipment. E.g. Vertical autoclave, CIP system
-
-
-
- It preserves the product status of raw material / In-process material /Product/QC samples e. g Nitrogen
-
-
-
- Produces data, which is used to accept/reject in-process material or product e.g. electronic batch recording system, critical process parameter chart recorder.
-
-
-
- It is a process control system that may affect product quality and for which alternative independent verification is not available e.g. PLC, SCADA, or DCS.
-
-
Equipment or system shall be defined as “Indirect Impact” if it meets any of the following criteria:
-
- Indirect impact equipment /systems will be those parts that are connected to direct impact or are playing role in “direct impact systems/equipment” e.g. Carton packing machine, Sticker labeling machine, etc.
-
- Equipment or system shall be defined as “No impact” has no impact on product quality e.g. lifting and positioning device, Conveyor belt, Shipper coding machine, etc.
-
- Appropriate qualification practices shall be applied to “Direct Impact” equipment or system, and “Indirect impact” equipment shall undergo enhanced commissioning.
-
- While the “No Impact” equipment shall be installed and commissioned according to “Good Engineering Practices”.
-
- All equipment and system are categorized and enlisted as direct impact, Indirect Impact, and No impact on the basis of Impact Assessment Exercise and attached as Annexure-6 ‘Categorization of equipment and system”.
-
- “Direct Impact” and Indirect Impact” equipment shall be subjected to periodic re-qualification/ validation as per the defined schedule in VMP.
-
User Requirement Specifications (URS) – Equipment:
-
- User requirement specification (URS) shall be prepared by the user department in co-ordination with engineering based on previous performance and practical experience.
-
- The following points shall be considered for the preparation of URS but not limited to:
-
-
- Objective
-
-
-
- Scope
-
-
-
- Responsibility
-
-
-
- Equipment / System description
-
-
-
- User requirement specification
-
-
-
- Equipment / System performance
-
-
-
- Material of construction
-
-
-
- Required documents
-
-
-
- Utility available on site
-
-
-
- Safety Design features
-
-
-
- Location and Environment
-
-
-
- Support required from the supplier
-
-
-
- Attachment(s)
-
-
-
- Abbreviation(s)
-
-
-
- Review and Comments
-
-
-
- Approval
-
-
- Approved URS shall be submitted to the Quality Assurance department for issuing an ‘UNCONTROLLED COPY’ to the Engineering/Project department for identification of vendor in co-ordination with the Purchase Department.
-
- If required a PDF version also provided to the Engineering department for necessary action with the purchase department and vendor.
-
- Based on the URS, the proposals given by the vendors are reviewed and the vendor who meets the specification is selected and referred to the purchasing department for the procurement.
-
Design Qualification (DQ) – Equipment:
-
- The DQ is aimed to specify that the equipment, system, or facility is designed in accordance with the requirements of the user and Good Manufacturing Practice (GMP).
-
- The design qualification document shall be prepared, reviewed, and approved by the Manufacturer.
-
- For feasibility, the DQ document is reviewed and approved by the User, Engineering, and QA department.
-
- If Design qualification is not provided by the manufacturer then the DQ document shall be prepared by the engineering department in coordination with the user department based on User Requirement Specifications and technical specifications provided by the manufacturer.
-
- The Design qualification document shall include the following details but not limited to:
-
-
- Pre-approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Overview
-
-
-
- Qualification Team and Responsibility
-
-
-
- Pre-requisites
-
-
-
- Equipment /System description
-
-
-
- Equipment /System/Technical Specification
-
-
-
- Review/evaluation of vendor design against user requirements
-
-
-
- Deficiency sheet
-
-
-
- Review Inclusive of follow up action (if any)
-
-
-
- Abbreviation(s)
-
-
-
- Recommendation/Conclusion
-
-
-
- Post-approval
-
-
- The DQ document must cover all the necessary diagrams, Layout, location Suitability desired special feature of components, equipment and their specification, desired material of construction, location of the control panel, electrical requirement and utility requirement
-
- After the signoff, any change in DQ of equipment which may have a direct or indirect impact on Quality, Safety, and Efficacy of the product shall be evaluated and incorporated through the change control procedure. Reference “SOP for Change Control Management“.
-
Factory Acceptance Test (Optional) – Equipment Qualification:
-
- For major and tailor-made equipment, Factory Acceptance Test (FAT) may be carried out by User/QA and Project/Engineering department through a FAT document which shall be provided by the vendor.
-
- The necessity of FAT shall be evaluated by the User, Project/Engineering, and QA Department.
-
- The Factory Acceptance Test shall be performed in supplier document and shall include the following (but not limited to):
-
- Visual inspection of components:
-
- This includes the verification of dimension, motor, blower specification, MOC, Valves (Size/No and safety requirements (alarm/Interlocks)
-
- Critical operational requirement:
-
- This includes the verification of critical operation based on URS
-
-
- Proof of functionality, by either a conventional function test or by simulation
-
-
-
- Verification of documents (availability and quality)
-
-
-
- Overall Review/Inspection
-
-
- Any modification with respect to the approved design, requirements shall be identified, and the same is explained to the vendor and documented as minutes of the meeting duly signed by User, QA Project/Engineering, and Vendor.
-
- Any modification from the approved DQ, identified during FAT shall be incorporated through Change Control and considered during Installation Qualification.
-
- The minutes of the meeting shall be annexed to the change control procedure.
-
- The FAT document shall be filed in the Quality Assurance department.
-
Site Acceptance Test (Optional) – Equipment Qualification :
-
- Site acceptance test (SAT) shall be performed by the User department, Engineering, and QA at the site after the recipient of the equipment/system in presence of the vendor to ensure that the equipment/system is in a good state, no components are damaged and meeting the designed as per URS.
-
- SAT involves inspection of equipment Major or sub-components in packed or unpacked condition, alignment checks of various components, systems, and subsystems.
-
- Master document –Maintenance /user manual, Qualification document (If applicable), calibration certificate.
-
- SAT also includes verification of supporting utilities, pendants availability, and adequacy, etc at the site.
-
- SAT done as per the checklist attached as Annexure- 7
-
Installation Qualification (IQ) – Equipment & System :
-
- The installation qualification (Equipment) document of equipment shall be prepared by the engineering department in coordination with the user department and Quality assurance.
-
- IQ is carried out to ensure that the premises supporting utilities and equipment have been built and installed in compliance with their approved design specification (DQ) and the manufacturer’s manual and recommendations.
-
- Installation qualification documents shall include the following details (but not limited to):
-
-
- Pre Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Equipment description & Identification
-
-
-
- Qualification team & responsibility
-
-
-
- List of reference documents & drawings
-
-
-
- Pre-requisites
-
-
-
- Checklist for Inspection of Equipment on receipt
-
-
-
- list of Spare parts received
-
-
-
- Location suitability
-
-
-
- Physical verification of area
-
-
-
- Verification of major component
-
-
-
- Verification of Installation
-
-
- Identification of equipment / Instrument for calibration and preventive maintenance
-
-
- Material of construction
-
-
-
- Utilities / Services connection checks
-
-
-
- Cleaning & Passivation
-
-
-
- Manufacturer Certificates
-
-
-
- Identification of product contact surfaces
-
-
-
- Deficiency sheet
-
-
-
- Attachment(s)
-
-
-
- Abbreviation(s)
-
-
-
- Summary and Conclusion
-
-
-
- Post Approval
-
-
- Wherever IQ documents are compiled by the vendor the document submitted by the vendor is evaluated and Any parameters,
-
- If not included in the document submitted by the vendor shall be carried out and details shall be documented.
-
- Any non-compliance observed during Installation qualification shall be recorded.
-
Operational Qualification (OQ) – Equipment and System:
-
- After the successful completion of IQ. OQ shall be performed to verify that the equipment, instrument, utility, and system, operates in accordance with design specification, manufacturer recommendation, and cGMP requirements.
-
- Operational qualification document of equipment shall be prepared by the User department In coordination with the engineering department and Quality Assurance.
-
- OQ of equipment shall be prepared based on the design qualification and manufacturer’s manual and recommendation.
-
- Operational Testing is to be done, whenever possible to challenge the system, to the limits of anticipated operating conditions.
-
- Typically OQ shall be done “without load”.
-
- However, if the equipment cannot be run without load, then load trials may be taken.
-
- OQ must also verify the performance of the equipment’s components, as applicable, such as motors, blowers, sensors, the functioning of interlocks, safety features, etc.
-
-
The Operation qualification document shall include the following details (but not limited to):
-
-
-
- Pre- Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Qualification team and Responsibilities
-
-
-
- Equipment operating principle
-
-
-
- Pre-requisites for operational qualification
-
-
-
- Methodology for operation qualification
-
-
-
- Calibration review of the critical instrument like a sensor, probes, gauges, recorders, airflow rates,
-
-
-
- Directions, pressure, temperatures, etc, And referred standard test instrument.
-
-
-
- Filter integrity and efficacy test
-
-
-
- Operational verification of Equipment i.e. Operational testing as per process & system
-
-
-
- Requirement and challenging
-
-
-
- Verification of draft SOP,s & Training
-
-
-
- Critical parameters of DQ verified in OQ.
-
-
-
- Testing of safety feature & interlocks
-
-
-
- Power failure verification
-
-
-
- Training to personnel for Maintenance and Operation
-
-
-
- Deficiency sheet
-
-
-
- Abbreviation(s)
-
-
-
- Attachment(s)
-
-
-
- Summary and conclusion
-
-
-
- Post Approval
-
-
- Wherever OQ documents are compiled by the vendor, the document submitted by the vendor is evaluated, accepted, and approved by Quality assurance.
-
- Any parameters, if not included in the document submitted by the vendor shall be carried out and details are documented.
-
- Any non-compliance observed during operational qualification shall be recorded.
-
- Operational qualification normally performed after IQ but depending on the complexity of the equipment, it may be performed as a combined Installation /Operational Qualification (IOQ).
-
- The completion of successful OQ should be allowed the finalization of standard operating and cleaning procedures, Operator training, and preventive maintenance requirement.
-
Performance Qualification (PQ) – Equipment and System:
-
- Performance qualification is the final stage of qualification, which demonstrates how the equipment/system will perform when challenged under simulated or actual production conditions.
-
- A series of tests are designed to demonstrate that the equipment/system is capable to perform consistently and meet required specifications under routine production operations.
-
- Studies on the critical variables shall be included a condition or a set of conditions encompassing upper and lower processing or operating limits and circumstances, commonly referred to as “worst case” conditions.
-
- Performance qualification document of equipment and system shall be prepared by QA (Validation) in coordination with the user department & Engineering department.
-
- Performance Qualification of equipment shall be prepared based on the user’s requirement and design qualification/technical specification, provided by the manufacturer.
-
-
The Performance qualification document shall include the following details (but it’s not limited to):
-
-
-
- Pre- Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- EquipmentQualification team and Responsibilities
-
-
-
- Pre-requisites for performance qualification of equipment
-
-
-
- Methodology for performance qualification of equipment
-
-
-
- Performance verification Tests
-
-
-
- Deficiency sheet
-
-
-
- Abbreviation(s)
-
-
-
- Attachment(s)
-
-
-
- Summary and Conclusion
-
-
-
- Recommendation
-
-
-
- Post Approval
-
-
- PQ shall normally the successful completion of IQ and OQ.
-
- However, it may in some cases be appropriate to perform it in conjunction with OQ or process validation.
-
- Any significant changes to the approved DQ/IQ/OQ/PQ documents during execution, e.g. acceptance criteria, operating parameters, etc., should be documented as a deviation and be scientifically justified.
-
- Conditional approval to proceed to the next qualification stage can be given where certain acceptance criteria or deviation have not been fully addressed and there is a documented assessment that there is no significant impact on the next activity.
-
- Qualification activities wherein locations need to be identified for placement of data logger or sensors, then schematic layouts to depicts the position of sensors or location identification shall be specified in the Qualification/validation protocol for better clarity.
-
- In some cases when the equipment operational ranges or any other additional checks are not validated during performance qualification then it shall be the part of process validation (PV).
-
- Any non-compliance observed during performance qualification shall be recorded.
-
- After Completion of qualification of new equipment/ system shall be released for routine activity after approval as per Annexure 13
-
Requalification of Equipment and System:
-
- The extent of requalification after the change shall be justified based on the risk assessment of the change.
-
- Requalification after the change shall be considered as part of the change control procedure.
-
- Re-qualification shall be carried out for one or more of the following reason, (but not limited to):
-
-
- Modification in the equipment which directly or indirectly affects the quality of the products being processed on the equipment
-
-
-
- Relocation of the equipment.
-
-
-
- Any other change as deemed necessary for requalification through the change management system.
-
-
-
- Equipment Up-gradation
-
-
- Note: Re-qualification shall not be required for portable equipment (Like Vibro sifter, Multi mill, Metal detector, Vibro deduster, Stirrer, Checkweigher )
-
- However, procedures for operating of this equipment shall be available that shall contain extensive checks on the equipment prior to its operation, and operation of any equipment must be verified prior to use.
-
-
During qualification and re-qualification activity following shall be considered :
-
-
-
- Acceptance criteria shall be adequately defined to enable configurations are attained.
-
-
-
- The approach taken towards qualification /validation activity (e.g. selection of product, test, and batch sizes for OQ/PQ) shall be based on rational /risk assessment.
-
-
-
- “Challenge test scripts” (additional protocols ) shall be used during qualification/validation.
-
-
- Referenced in the qualification documents
-
- Pre-approved before starting activities
-
- Descriptive & clear so as to avoid ambiguity.
-
- The re-qualification document shall include all essential qualification details as per initial Installation, Operational, and Performance qualification content.
-
- The re-qualification documents shall be prepared separately (RIQ, ROQ, and RPQ or common requalification document (RQ) shall be prepared for equipment or system after change.
-
- Re-qualification document shall be prepared as per Annexure -2 and the content of the document shall be as per type document i.e. RIQ, ROQ, RPQ, and RQ, etc.
-
-
Periodic re-qualification of equipment:
-
-
- Periodic re-qualification of “Direct Impact” and Indirect Impact” equipment’s shall be carried out as per schedule defined in the VMP.
-
-
- For Direct impact Equipment: 3 years ± 3 month
-
-
-
- For Indirect impact Equipment: 5 years ± 3 months
-
-
- Periodic re-qualification documents shall be prepared by QA in coordination with engineering and User.
-
- The periodic re-qualification documents shall include the following details:
-
-
- Pre-Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Qualification team and Responsibilities
-
-
-
- Equipment Description
-
-
-
- Reference Documents
-
-
-
- Pre-requisites for equipment re-qualification (Review of change control, Document Verification, Physical verification of equipment, PM detail verification, Breakdown details verification, Calibration verification)
-
-
-
- Installation Verification
-
-
-
- Operational Verification
-
-
-
- Performance Evaluation
-
-
-
- Details of non-conformance
-
-
-
- Summary and Conclusion
-
-
-
- Recommendation (S)
-
-
-
- Abbreviation(s)
-
-
-
- Attachment(s)
-
-
-
- Post-approval
-
-
-
Dismantling of Equipment:
-
-
- For Dismantling of the Equipment/system, the request shall be raised as per the Annexure- 3 (Equipment/Instrument/Utility retirement form)
-
- Dismantling verification document shall be prepared by the User department in coordination with Engineering and QA for equipment whenever equipment has to be transferred or removed from its qualified location.
-
- The dismantling document shall be prepared as per the Annexure-2
-
- Dismantling verification of equipment shall be done whenever equipment has to be transferred or removed from the qualified location.
-
- Dismantling verification document shall include the following details (but not limited to):
-
-
- Pre- Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Verification team and Responsibilities
-
-
-
- Methodology for Dismantling verification
-
-
-
- Equipment description
-
-
-
- Dismantling procedure (Equipment verification, Document verification, Instrument calibration verification, Checks and tests to be performed before dismantling, Dismantling checks)
-
-
-
- Deficiency sheet
-
-
-
- Abbreviation(s)
-
-
-
- Attachment(s)
-
-
-
- Summary and Conclusion
-
-
-
- Post Approval
-
-
Qualification of Facility /Building
-
- Facility/building qualification shall be carried for new facilities/building, in order to ensure that the facility is according to the design specification and complying with the requirement of product, process, cGMP requirements, safety, and regulatory bodies.
-
- Facility qualification shall be initiated after the protocol for facility qualification is approved & signed.
-
- During facility qualification, emphasis shall be given to below mention parameter but not limited to :
-
-
- Compliance to cGMP with respect to the facility as defined in the guideline of National and international regulatory bodies
-
-
-
- Floorwise approved layout of the facility.
-
-
-
- Design criteria of the rooms
-
-
-
- Room area in square feet as designed.
-
-
-
- Type of construction & related certificate
-
-
-
- Floor, wall & ceiling construction, their finish & type of coving
-
-
-
- Door and window fitting, Door closures, view panels, vision panels, Interlocks
-
-
-
-
- Type of electrical fitting and finish
-
-
-
-
-
-
- The drainage system and the type of drains designed
-
-
-
-
-
-
-
- Fire and safety alarm system
-
-
-
-
-
-
-
- Manufacturing licenses from Local FDA
-
-
-
-
-
-
-
- Environmental conditions like temperature, RH, and pressure differential condition.
-
-
-
-
-
-
-
- Cleaning and sanitization of area.
-
-
-
-
-
-
-
- Performance of HVAC system
-
-
-
-
-
-
-
- Approved and release of facility/ building for routine operation.
-
-
-
-
-
Miscellaneous/ General Validation study document:
-
-
- The document numbering system for miscellaneous validation study shall be maintained as per Annexure- 4
-
- Document for miscellaneous study shall be prepared as per the Annexure-14and content of study protocol/ report shall be as per study purpose.
-
- Miscellaneous validation study document shall be prepared by the User department
-
- Miscellaneous study document shall Include the following details (but not limited to):
-
-
- Approval
-
-
-
- Objective
-
-
-
- Scope
-
-
-
- Responsibilities
-
-
-
- Methodology for study
-
-
-
- System description
-
-
-
- Study procedure
-
-
-
- Deficiency sheet
-
-
-
- Abbreviation(s)
-
-
-
- Attachment(s)
-
-
-
- Summary and Conclusion
-
-
-
- Recommendation
-
-
- The miscellaneous study protocol numbering log shall be maintained as per the Annexure-9.
-
- The miscellaneous study protocol numbering log shall be maintained as per the Annexure-9.
6.0 Reference (S) – Equipment and System Qualification :
-
- Validation Master Plan (VMP)
-
- ICH, (CDER and CBER), Q9 Quality risk management, Guidance for industry, June 2006
-
- EU guidelines for GMP for Medicinal products for human and veterinary use, Annex 15: Qualification and Validation
7.0 Glossary :
| SOP | : | Standard Operating Procedure |
| QA | : | Quality Assurance |
| DQ | : | Design Qualification |
| PQ | : | Performance Qualification |
| IQ | : | Installation Qualification |
| OQ | : | Operational Qualification |
| URS | : | User Requirement Specification |
| Sr. | : | Senior |
| MOC | : | Material of Construction |
| PLC | : | Programmable Logic control |
| SCADA | : | Supervisory Control and data Acquisition |
| DCS | : | Direct control system |
| VMP | : | Validation Master Plan |
| GMP | : | Good Manufacturing Practice |
| PM | : | Preventative maintenance |
| HVAC | : | Heating Ventilation and air Conditioning |
| OSD | : | Oral Solid Dosage |
| PDL | : | Product Development Lab |
| FAT | : | Factory acceptance test |
| SAT | : | Site Acceptance test |
| FDS | : | Functional design specification |
| IOQ | : | Installation Operational qualification |
| RQ | : | Re-Qualification |
| PRQ | : | Periodic re-qualification |
| RIQ | : | Re-installation Qualification |
| ROQ | : | Re-Operational qualification |
| RPQ | : | Re-Performance qualification |
8.0 Annexure – Equipment and System Qualification:
Annexure-1- Equipment Qualification/Requalification schedule
Document No.: ________ Department:_______ Section:_______Effective Date:______
|
Sr. No. |
Name of Equipment | Equipment ID. No. | Initial Qualification Date |
Qualification/ Requalification Done On |
|
Periodic Re-qualification due on |
Requalification
Completion Date |
Next Re-Qualification
Due on |
Checked By
(Sign./Date) |
Remarks |
Annexure-2- Format for preparation of qualification documents (specimen copy)
QUALIFICATION DOCUMENT OF —————————-
| DEPARTMENT | : | |
| LOCATION | : | |
| NAME OF THE EQUIPMENT | : | |
| EQUIPMENT I. D. | : | |
| MAKE | : | |
| MODEL / TYPE | : | |
| EFFECTIVE DATE (POST APPROVAL) | : |
| Document No. & Rev. No. | Effective Date (Pre-approval) | Reason for revision |
TABLE OF CONTENTS
| Sr. No. | Content | Page No. |
| 1. | ||
| 2. | ||
| 3. |
Annexure-3- Equipment / Instrument/Utilities Retirement Form
|
EQUIPMENT / INSTRUMENT / SYSTEM DETAILS |
| Date :
Name of the instrument/equipment/system : Instrument/ equipment ID : Make : Model : Sr. No : Area of installation : Reason for Retirement/Removal: (Attach additional sheet if required)………………………………… Dept. Head: (Sign/date) (Sign/date) |
| QA Comments |
| Remarks: (Attach additional sheet if required)……………………………………………………….
QA Manager (Sign/date) |
| Approval |
| Remarks: (Attach additional sheet if required)………………………………………………………………………………
QA Head (Sign/date) |
Annexure-4- Qualification Document Numbering System
Refer As per respective SOP for Numbering System
Annexure-5- Traceability Matrix
| Equipment Name: | Equipment ID No.: | Location: | ||||||
| Make: | Model: | Sr. No.: | ||||||
| Sr. No. | URS No. | DQ | FAT | SAT | IQ | OQ | PQ | Remarks |
Annexure-6- Categorization of equipment and system
Production Equipment
A) Equipment having “Direct Impact “on the product Quality:
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Sifter | 2. | Multi mill |
| 3. | RMG | 4. | FBD |
| 5. | Tray Dryer | 6. | Compression Machine and line equipment |
| 7. | Blender Bin | 8. | Capsule filling machine and line equipment |
| 9. | Coating machine | 10. | Alu-Alu Packing Machine |
| 11. | Blister Packing Machine | 12. | Storage Vessel |
| 13. | Strip Packing Machine | 14. | Basket Filter |
| 15. | Manufacturing Vessel | 16. | Inline Homogenizer |
| 17. | Sugar Dissolving Vessel | 18. | Bottle Washing Machine |
| 19. | Filter press | 20. | 2 D Vision and printing system |
| 21. | Filling & Sealing Machine | 22. | Inspection belt (manual) |
| 23. | Stirrer | ||
B) Equipment having “Indirect Impact “on the Product Quality:
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Jacketed Vessel or Paste kettle or Solution preparation tank | 2. | Jet Cleaning Machine |
| 3. | Checkweigher | 4. | Carton packing machine |
| 5. | Optical Inspection Machine(Manual) | 6. | IR Dryer |
| 7. | Dedusting booth | 8. | Sticker labeling machine |
| 9. | Camera Inspection Machine | ||
C) Equipment having “No Impact “on the Product Quality :
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Lifting and Positioning device | 2. | Conveyor belt |
| 3. | Turntable | 4. | BOPP Taping Machine |
| 5. | SS Pressure Vessel | 6. | Strip de-foiling machine |
| 7. | Stacker and lifter | ||
Utility Equipment And System
A) Equipment/system having “Direct Impact “on the Product Quality:
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Air Handling Unit (Classified areas) | 2. | AHU cum Dehumidifier |
| 3. | Dehumidifier unit | 4. | Purified water generation, storage and distribution system |
| 5. | Compressed air system | 6. | Nitrogen system |
B) Equipment/System having “Indirect Impact “on the product
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Dry Scrubber | 2. | Wet scrubber |
| 3. | Filter cleaning machine | 4. | Dust Extraction Unit |
C) Equipment/System having “No Impact “on the Product Quality:
| Sr. No | Equipment Name | Sr. No | Equipment Name |
| 1. | Boiler | 2. | Chiller |
| 3. | D.G Set | 4. | Hot water generator |
| 5. | Cooling tower | 6. | Storage tanks (other than water system) |
| 7. | Vacuum system | 8. | Ventilation supply |
| 9. | Ventilation exhaust | 10. | Dust collector |
| 11. | Air conditioners | 12. | ETP Plant |
| 13. | Electrical system | ||
Annexure-7- Site acceptance test format
| Sr. No. |
Job Description |
Status | Observation if any | Remarks |
| 1. | Dimension as per drawing. | OK/Not OK | ||
| 2. | MOC Certificate of product. | O/Not OK | ||
| 3. | Surface finish certificates if applicable. | Available/ Not Available | ||
| 4. | Motors, Gearbox or other parts should be of Standard make. | OK/Not OK | ||
| 5. | Service manuals along with control Drawings are provided. | Available/ Not Available | ||
| 6. | Others | OK/Not OK | ||
| Comments if any: | ||||
Annexure-8- Time Based activity schedule
| Sr. No. | Validation for | Area | Frequency | Last done date | Scheduled on |
| Done date | Next
Due on |
updated By
(Sign./Date) |
Checked By
(Sign./Date) |
Remarks |
Annexure-9- Miscellaneous study protocol numbering log
| Sr. No. | Protocol Name | Protocol No. and Effective date | Updated by
(Sign./Date) |
Checked by
(Sign./Date) |
Report No. & Effective date | Updated by
(Sign./Date) |
Checked by
(Sign./Date) |
Remark |
Annexure-10- Recommendation format
Recommendation for…………………………………..
Ref. Protocol No. & Revision No.: Effective Date:
Report No. & Revision No.: Effective date:
Batch size:
Recommendation:
Annexure-11-Addendum to Validation document format.
Addendum to ……. .…….
Addendum No.: Effective Date
Initial Document Name:
Initial Document No:
Effective Date of Initial Document:
Description of Supplement:
Annexure-12- Qualification document numbering log
| Sr. No. | Equipment Name | Equipment ID | URS No. | DQ No. | IQ No. | OQ No. |
| PQ No. | Updated by (Sign./Date) | Checked by (Sign./Date) | Remark |
Annexure-13- Release of Equipment
Release of Equipment/System for Routine Activity
This is to certify that…………………………….
Name of the Equipment/System: Equipment ID:
Make: Model:
Has been Installed, Commissioned, and Qualified in …………area having room ID …… and same has proven satisfactory.
Thereby, the Equipment/ System is handover to the user department on
Date …………..
Recommendation:…………………………………………………………………………………………
Annexure-14- Format for preparation of Miscellaneous validation/ qualification study documents
VALIDATION /QUALIFICATION STUDY DOCUMENT OF —————————-
| Document No. & Rev. No. | Effective Date | Reason for revision |
*********************************************END*********************************************


