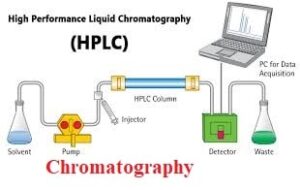
Chromatography is a laboratory technique for the separation of a mixture. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate.
GOOD CHROMATOGRAPHY PRACTICES
1.0 PURPOSE :
-
- The purpose of this Standard Operating Procedure (SOP) is to describe the procedure of Good Chromatographic Practices.
2.0 SCOPE :
-
- This SOP shall be used as such for “Good Chromatographic Practices” in Quality Control Laboratory.
3.0 RESPONSIBILITY – GOOD CHROMATOGRAPHY PRACTICES
-
- The Executive/Officer QC shall be responsible;
- The Executive/Officer QC shall be responsible;
-
- To carry out the analysis and record the findings as per SOP.
-
- To intimate the Head QC / designee in case of any deviation from the SOP.
-
- The Section head / Manager QC shall be responsible;
-
- Ensure implementation and adherence to the system as per the SOP.
-
- Evaluate proper documentation.
-
- Provide training as and when required.
-
- The Head QA/his or her designee shall be responsible for;
-
- To ensure implementation and adherence to the system as per the SOP.
4.0 PROCEDURE – GOOD CHROMATOGRAPHY PRACTICES
- Precautions during Chromatography:
-
- Indicate on the status board, the name of product/ material, batch number / A.R.No., Stage, Test, Start time, and Analyst (Sign/Date) prior to starting the chromatography.
-
- Make the relevant entry in the chromatography instrument logbook.
-
- Handle the chromatography column with extreme care.
-
- Always keep both ends closed after usage.
-
- Increase/ decrease the mobile phase flow rate stepwise and slowly.
-
- Use always filtered degassed Chromatography (HPLC) grade solvents/ mobile phases.
-
- Do not overtighten fittings of the injector, column, pump, or detector.
-
- Ensure that the date and time of data acquisition appear on each chromatogram and the chromatograms are compiled in succession.
Related: SOP for Waters HPLC system with Empower Software
-
- System suitability parameters shall be checked by the analyst before proceeding with the sample analysis.
-
- All solutions shall be clear homogeneous and free from particulate matter.
-
- Filter the solutions before use.
-
- While going through change over from reverse phase to normal phase and normal phase to reverse phase follow the changeover steps.
-
- Use High-Performance Liquid Chromatography (HPLC) grade solvents
-
- In case of non-availability of Chromatography, grade solvents use AR grade solvents
-
- Use Elga/MilliQ or any high purity water (suitable for chromatography) for the preparation of the mobile phase.
-
- Never mix the aqueous phase and organic phase portion in measuring cylinder as it can give erroneous composition.
-
- Column shall be washed pre and post running of the sample set.
-
- It should be part of the Sample set/sequence.
-
- Flush the High-Performance Liquid Chromatography (HPLC) system with hot water (Approx. Temp 50-600C or as per the suitability of tubing) by using union in place of Column at least by weekly.
-
Preparation of mobile phase and usage of solvent for Chromatography :
-
- If the mobile phase contains a buffer solution, first calculate the quantity of the mobile phase required for complete analysis (including quantity required for dilution) and prepare the buffer solution as per the procedure.
-
- Set the pH (If required) and do not use concentrated acid/alkali directly for pH adjustment,
-
- Use diluted acid/alkali solution for pH adjustment.
-
- Filter the buffer solution through 0.45µ nylon filter or as mentioned in respective STP.
-
- The filter also can be done at the time of the final mobile phase composition, if applicable.
-
- Measure the aliquot of buffer solution required in the mobile phase composition.
-
- Transfer to a clean and dried stoppered bottle.
-
- Measure the required quantity of organic solvents separately and
-
- Add to the stoppered bottle containing a buffer solution and mix well.
-
- If the mobile phase contains a small amount (5% or less) of solvents,
-
- use a volumetric flask/pipette/measuring cylinder of appropriate size for the measurement.
-
- Degas by sonication or vacuum for 4-5 minutes.
-
- Do not degas the mobile phase for a longer time containing organic solvents.
-
- It may alter the composition while sonication ensures that the bottle cap is loosened to avoid the pressure built up.
-
- If the mobile phase is reverse-phase then rinse the filtration assembly and
-
- Collection vessel with water before filtration followed by the mobile phase.
-
- If the mobile phase is a normal phase then rinse the filtration assembly and collection vessel with the water-miscible component of the mobile phase before filtration followed by the mobile phase.
-
- Do not sonicate the buffer solution prepared by using acetate and phosphate buffer since on sonication it forms the complex which may interfere with the analysis.
-
Usage of the chromatography column, System set-up, and Sample analysis:
-
- Physically check the column intactness and then Connect the column on HPLC.
-
- Wash the column with appropriate solvents and
-
- Saturate the column with the mobile phase for about 30 minutes or more until the baseline gets stabilized.
-
- The injection sequence (sample set) shall be prepared by the analyst for the respective tests.
-
-
The injection sequence shall contain the following but not limited to:
-
-
-
- Vial No.
-
-
-
- Injection volume.
-
-
-
- Sample name (sufficient details to link each chromatogram with the sample, like Product name, B. No./AR No., Stage).
-
-
-
- Method set name.
-
-
-
- Chromatogram No./Data No./ Injection No. etc.
-
-
- Before starting the sequence, the reviewer or section head shall ensure that the sequence (sample set) parameter and respective instrument method parameters are as per STP/GTP.
-
- There shall not be any trial injection run to check the suitability of the system (except System Suitability run defined in respective STP/GTP).
-
- All injections shall be run as a part of the main sequence.
-
- Check that the
-
-
- Peak shape,
-
-
-
- Retention time,
-
-
-
- Relative retention time
-
-
-
- Resolution,
-
-
-
- Asymmetry,
-
-
-
- System pressure and theoretical plates, etc. from the system suitability run / First run of Standard preparation and
-
-
- If required to make necessary modification in the system as per “Allowable modification in chromatography system”,
-
-
The modification shall not be made prior to Authorization.
-
-
- After satisfactory system suitability run (if applicable) inject blank (i.e. diluents, mobile phase, etc.) standard solution, check the system suitability parameters, and if it meets then start sequence.
-
- If peak splitting or broadness of peak occurs during the analysis stop the analysis, then after proper washing of column bracketing standard injection/system suitability solution shall be injected.
-
- If it meets with the system suitability then analysis shall be carried on from that sample where peak splitting or broadness took place.
-
- Fill the details of system suitability parameters in the respective analytical template/worksheet.
-
- After completion of the analysis, wash the column with an appropriate solvent for the appropriate time (e.g. Column shall be washed with water for a long time if the buffer concentration is more in the mobile phase and/or if the column is used for a long time), rinse/ purge auto-injector and make necessary entry in the “Column usage log” and in “Instrument usage logbook”.
-
- All chromatograms shall be part of the final reports.
-
- Ensure the pressure graph/run shall be enabled while creating/modifying the instrument method.
-
Handling of chromatography column change while analysis :
-
- If the peak shape is not satisfactory system suitability parameters are not achieved as per the limit even after making necessary modifications in the system, the column can be changed with the following documentation.
-
-
- Make entry of previously used column in the column usage log with the reason of discontinuation and keep for washing.
-
-
-
- Take the print of all chromatograms generated on the previous column and put the reason for column change on the Chromatogram checklist along with the previous column no.
-
-
-
- Attach all chromatograms with the relevant document / Template / Analytical report/worksheet.
-
-
Handling of mobile phase change while chromatography analysis:
-
- If the peak shape is not satisfactory or resolution is not achieved or the theoretical plate and/or tailing factor is not within the limit even after making necessary modifications in the system mobile phase can be changed with the following documentation.
-
-
- Make an entry in the instrument usage logbook with the reason for discontinuation of the mobile phase.
-
-
-
- System suitability check is a must for every new mobile phase and column.
-
-
-
- The mobile phase cannot be added in between the analysis of the running mobile.
-
Note A) ± 10% flow rate adjustment in RT shall be considered during the analysis.
B) ± 1-minute tolerance shall be considered for retention time up to 10 minutes, ± 10% tolerance shall be considered for retention time more than 10 minutes.
-
Allowable modification in Chromatography system:
-
- Adjustments to the specified chromatographic system may be necessary in order to meet system suitability requirements.
-
- Chromatographic systems adjustments performed in order to comply with system suitability requirements are not to be made in order to compensate for column failure, Analytical error, and system malfunction.
-
- Adjustments are permitted only when suitable standards (including Reference Standards / Working Standard) are available for all compounds used in the suitability test; and
-
- The adjustments or column change yields a chromatogram that meets all the system suitability requirements specified in the official procedure.
-
- If adjustments of operating conditions are necessary in order to meet system suitability requirements,
-
- Each of the items in the following list is the maximum variation that can be considered unless otherwise directed in the ATP.
-
- Multiple adjustments can have a cumulative effect on the performance of the system and are to be considered carefully before implementation.
-
- In some circumstances, it shall be desirable to use an HPLC column with different dimensions to those prescribed in the official procedure (different length, internal diameter, and/or particle size).
-
- In either case, changes in the chemical characteristics (“L” designation) of the stationary phase will be considered a modification to the method and will require full validation.
-
- Adjustments to the composition of the mobile phase in gradient elution may cause changes in selectivity and are not recommended.
-
- The adjustments are allowed only to improve the quality of the chromatography unless otherwise directed in the respective pharmacopoeial monograph/GTP/STP.
Note: Modification in the allowable chromatographic system shall be within the raggedness study performed during the method validation study.
-
- Any adjustment done shall be part of the reporting.
-
- The dis-positioning of the batch/sample will be subject to the approval of this report.
-
- Alternate columns (Different make) can be used in case of column fails to meet the system suitability requirement.
-
- But it should be defined in respective GTP/STP and also covered under AMV / Pharmacopoeial evaluation study.
-
- The following are the general criteria, which provide the extent of allowable variation to get the system suitability.
-
-
The pH of Mobile phase:
-
-
- The pH of the aqueous buffer used in the mobile phase preparation can be adjusted to within ±2 pH units.
-
- Example: If the specified pH is 7.0 then the allowable limit for adjustment is 6.80 – 7.20.
-
-
The concentration of Salts in Buffer:
-
-
- The concentration of salts used in the preparation of aqueous buffer used in the mobile phase can be adjusted within ±10%
-
- (Ex.: If the specified concentration is 1.0% then the allowable limit for adjustment is 0.90 % – 1.10 %).
-
-
Stationary phase in chromatography :
-
-
- Column length: ±70%.
-
- (Ex.: If specified length is 25 cm then allowable limit for adjustment is 7.5 cm – 42.5 cm).
-
- Column internal diameter: ±25%
-
- (Ex.: If specified internal diameter is 4.6 mm then allowable limit for adjustment is 3.45 – 5.75 mm.).
-
- Particle size :
-
- A maximum reduction of 50%, no increase permitted.
-
- (Ex.: If specified particle size is 5 micron then the allowable maximum reduction is 2.5 micron).
-
- Flow rate: When column dimensions have been modified, the flow rate can be adjusted using
-
- F2= F1 (L2 D2/ L1d2 )
-
- Where,
- F1: Flow indicated in the monograph in ml/min,
- F2: Adjusted flow rate, in ml/min,
- L1: Length of column indicated in the monograph,
- L2: Length of column used,
- d: Column inner diameter of the column indicated in the monograph
- D: Internal diameter of the column used
- Additionally, the flow rate can be adjusted ± 50 %.
-
- Column temperature: ±10%
-
- (Ex.: If specified column temperature is 40°C then allowable limit for adjustment is 36° – 44°C).
Note: If the column temperature and sample temperature are not mentioned in the ATP/STP, or it is mentioned to keep it “Ambient”, then in both cases the column temperature shall be set to 25°C and sample temperature to 15°C.
-
- Detector wavelength:
-
- Deviations from the wavelengths specified in the method are not permitted.
-
- Injection volume:
-
- The injection volume can be reduced/increased as far as is consistent with accepted precision and detection limits.
-
-
Mobile phase composition can be changed in chromatography as follows:
-
-
- The following adjustment limits apply to minor components of the mobile phase (specified at 50% or less).
-
- The amount(s) of these component(s) can be adjusted + 30% relative.
-
- However the change in any component cannot exceed + 10% of absolute (i.e. in relation to the total mobile phase), nor can the final concentration of any component be reduced to zero.
-
- Examples of adjustments are given below.
-
- The specified ratio of 50:50:
-
- Thirty percent of 50 is 15% absolute, but this exceeds the maximum permitted change of + 10% absolute in either component.
-
- Therefore, the mobile phase ratio may be adjusted only within the range of 40:60 to 60:40.
-
- The specified ratio of 60:35:5:
-
- For the second component, 30% of 35 is 10.5% absolute, which exceeds the maximum permitted change of + 10% absolute in any component.
-
- Therefore the second component may be adjusted only within the range of 25% to 45% absolute.
-
- For the third component, 30% of 5 is 1.5% absolute.
-
- In all cases, a sufficient quantity of the first component is used to give a total of 100%.
-
- Therefore, a mixture range of 50:45:5 to 70:25:5 or 58.5:35:6.5 to 61.5:35:3.5 would meet the requirement.
-
Duplicate standard / Similarity Factor calculation
-
- The duplicate standard shall be applied for the Assay test (irrespective of sample category).
-
- 2nd Standard shall be injected in duplicate after System suitability run (After completion of 5/6 replicate standard injection and prior to the sample run).
-
- Assay analysis shall be performed using duplicate standard preparation.
-
- The second standard shall be prepared by the same/different analyst.
-
- The first standard (Initial standard) shall be injected as per the above schedules, while the second standard shall be injected in duplicate.
-
- The correlation between two standards shall be calculated as per the below formula.
-
-
- Mean area of std -2 x Weight of std -1
- Mean area of std -1 x Weight of std -2
-
-
- Acceptance criteria: Between 0.98 -1.02
-
- In case of correlation does not fall within acceptance criteria,
-
- log the Lab Incident/Event as per the current version of SOP – Lab Incident, investigate and repeat the analysis by preparing the standard and establish the similarity factor prior to sample injection.
-
- For calculation mean area of the first standard shall be considered.
-
Bracketing standard procedure in Chromatography:
-
- System suitability shall be established as per the test procedure.
-
- After every defined sample injections or after every test (Club analysis or individual analysis) standard preparation in single shall be injected (Called as Bracketing standard preparation).
-
- Bracketing Standards shall be injected after 12 injections or 3 hrs after the last standard injection injected whichever is earlier.
-
- Bracketing standard can be injected other than the above conditions (Completion of Test, After 3 Hrs, After 12 sample injection), It should be justified and documented.
-
- To meet the system suitability criteria of method %RSD of last five injections (including Bracketing Standard) area to be considered,
-
- the average area including bracketing standard preparations shall be used in the calculations.
-
- For e.g. If System suitability is established by five injections of standard injection (1st, 2nd, 3rd, 4th, and 5th ).
-
- After testing the sample (A) preparations (6th and 7th ) and one bracketing standard preparation injection (8th ) shall be injected.
-
- Then further sample (B) preparation injections ( 9th and 10th ) followed by bracketing standard (11th ).
-
-
To calculate the result (A)
-
-
- Standard Avg. Area = Avg. Area of std (2nd, 3rd, 4th 5thand 8th).
-
- Sample(A) Avg. Area = Avg. Area of Sample (6th and 7th)
-
-
To calculate the result (B)
-
-
- Standard Avg. Area = Avg. Area of std (3rd,4th,5th, 8th, and 11th ).
-
- Sample(B) Avg. Area = Avg. Area of Sample (9th and 10th).
-
- The analysis is valid only if the %RSD of Bracketing Standard preparations are within the limit.
-
- If the RSD of bracketing standard is failed, stop the analysis,
-
- The analyst shall log the Lab Incident (as per the current version of SOP for Lab Incident) and investigate the reason for failure, adopt the strategy as follows.
-
- If the failure in RSD is because of system instability, bracketing standard RSD shall be considered up to last bracketing standard till it meets the criteria of system suitability and analysis of remaining samples shall be carried after reestablishing the system suitability as per the GTP.
-
- If there is a significant change in one of the injection area of bracketing standard and analysis is continued.
-
- Additional injections of bracketing standard shall be done and reason for variation in the previous injection of bracketing standard shall be justified.
-
- In case of vial missing/solution volume inside the vial is less than required, area variation between replicate injection, additional peak observed, re-injection from the same vial, or from the same solution shall be done, with proper scientific justification.
-
- Additional injection of the Blank-mobile phase and/or diluent, placebo preparation, and impurity standard can be injected to verify the elution pattern and peak identification of Blank, placebo, and impurity.
-
- Data shall be attached to the Analytical Report with scientific rationales.
-
Chromatogram set up for integration, review, Calculation, and Documentation:
Note1: Chromatograms shall be processed within one working day from the completion of the sequence. If exceeds, Log the Lab incident, Investigate / Justify and then process the acquired data.
Note 2: No single chromatogram shall remain unprocessed irrespective of Blank, Placebo, System check, standard, sample, etc.
-
- Analysts shall ensure the peak shape and system suitability parameters for all chromatograms in a sequence (i.e.Up to the last chromatogram) before the set up of integration parameters.
-
- Set the integration parameters like width, threshold, peak area, peak height, scale are selected appropriately for proper peak marking and detection and inhibit all others peaks except the principal peak in all tests except for related substance test and degradation product, Chromatography purity (but not limited to).
-
- Integration parameters shall be the same for all chromatograms generated in a sequence or test.
-
-
Manual Integration is not allowed.
-
-
- In case of the integration of peak is not possible by software, manual integration can be done for the proper peak integration with justification (Only in RS test).
-
- If different processing methods (Integration parameters) used in the same sequence then it shall be properly justified.
-
- In the system, suitability chromatogram identifies the peaks, its RRT, System suitability parameters, peak shape, and report the values as applicable.
-
- Identify each peak for RS test e.g.
-
-
- Blank-Diluent,
-
-
-
- Placebo,
-
-
-
- Principal peak,
-
-
-
- Unknown impurity,
-
-
-
- Known impurity (mention the name of impurity), etc. and
-
-
- Inhibit those peaks in the sample whose responses are similar in placebo and blank.
-
- If the response of an unknown peak in sample chromatogram is greater than the response of that peak in blank or placebo chromatogram then integrate that peak in both (sample and blank or placebo) chromatograms and consider area for calculation after (area of peak in sample-area of the peak in blank or placebo).
-
- In the case of the RS test, attach the overlay chromatogram of the sample, blank, and placebo for clarity of peaks.
-
- In-case of In-house product/ material if system suitability parameters ( theoretical plates, resolution, and tailing, etc.) do not comply as per acceptance criteria but peak shape or peak elution pattern is good then send all relevant data to the analytical method development team for to review and revise the system suitability acceptance criteria.
-
-
Chromatography Report Format :
-
-
- The custom report shall cover the following information but not limited, which is for information and can be modified as per the specific need.
-
-
- Name of Product / Raw Material
-
-
-
- Test performed
-
-
-
- Sample ID -B. No. / A.R. NO.
-
-
-
- Data Path
-
-
-
- Injection volume
-
-
-
- Vial no.
-
-
-
- Column number / ID
-
-
-
- WaveLength.
-
-
-
- Date of acquisition
-
-
-
- Date processed
-
-
-
- Acquired by /Analyst
-
-
-
- Instrument ID
-
-
-
- Chromatogram No / Data No / Injection No.
-
-
-
- Instrument method ID
-
-
-
- Processing method ID
-
-
-
- Print date / Time & Time Zone
-
-
- The peak table in the custom report shall cover the following data (But not Limited to), however other data as per requirement.
-
-
- Peak Name
-
-
-
- Retention time
-
-
-
- Area / Height
-
-
-
- Area% / Height%
-
-
-
- Tailing factor or Asymmetry
-
-
-
- Theoretical plates / Plate count
-
-
-
- Integration type
-
-
-
- Other system suitability parameter as per requirement
-
-
- Take the print out of integration parameters and all chromatograms.
-
- If Degradation / Related substances are to be calculated from Assay, take the separate print out of the first injection of each sample chromatogram by setting the width and threshold appropriately to detect all peaks along with blank or placebo.
-
- Report the system suitability data (theoretical plate, tailing factor, capacity factor, etc.) in the respective analytical template.
-
- In cases where reintegration is necessary, all the chromatograms from the previous integration or multiple integrations shall be identified, assessed, justified and shall be part of the raw data and set of chromatograms (previous Processing method print out, audit trail (for that specific change) and the print out shall be attached with the sequence of record).
-
For related substances and similar low content test.
-
- The analyst shall be zoomed the chromatograms on the baseline and shall check that all the peaks are integrated and the integration of all interested peaks are properly marked.
-
- Peaks that are not separated completely shall be integrated valley-to-valley extrapolation (tangential skim).
-
- The integration parameters shall set in such a way that the peak of at least half of the disregard limit must be integrated, and shall be documented in the Chromatogram checklist.
-
- The scale of chromatograms shall be set properly so that the peak shape of all interesting peaks and their integration can be seen clearly.
-
- In the chromatogram peak shall be identified by RT only and other detail like peak identification, etc. shall be part of the peak table.
-
- The sample chromatogram shall overlay with diluent and placebo chromatograms to identify the interested peak properly.
-
For Assay, Content uniformity, dissolution, preservative content:
-
- Integration shall be set appropriately for the principal peak.
-
- The scale of chromatogram shall be such that the response of principal peak is at least 70% of the full-scale deflection or Autoscale of the chromatograms.
-
- In the related substances/chromatographic purity/degradation test exclude the area of diluent/placebo peak in the impurity calculation.
-
- Where disregard peak/area is mentioned in GTP (based on LOQ performed during AMV), those peak/ peaks area shall be ignored in the calculation.
-
- In the calculation of impurity disregard the peak of response below 0.03% with respect to the principal peak where LOQ is not available.
-
- Calculate the area equivalent to 0.03% as follows :
-
-
For area normalization method :
- = 0.03 x area of the principal peak in sample preparation-1
- 100
-
For the external standard method :
- = 0.03 x X x Z
- Y x P
- Where,
-
-
-
- X= Concentration of drug substance (Theoretical) in sample preparation.
-
-
-
- Y= Concentration of external standard in standard preparation.
-
-
-
- Z= Area of external standard in standard preparation.
-
-
-
- P= Potency of an external standard.
-
-
- In the calculation of known and unknown impurity disregard the peak of response below LOQ level.
-
- Calculate the area equivalent to LOQ as follows.
- = L x A
- C x P
- Calculate the area equivalent to LOQ as follows.
-
- Where,
-
-
- L= LOQ of impurity in ppm
-
-
-
- C= Concentration of impurity in ppm from Standard preparation
-
-
-
- A= Area of impurity from standard preparation
-
-
-
- P = Potency of impurity standard
-
-
- In the calculation of known and unknown impurity disregard the peak of response below LOQ level.
-
- Where impurity standard is not injected and LOQ and RF are mentioned in GTP/Protocol.
-
- Calculate the area equivalent to LOQ as follows :
-
-
- L x A x RF
- C
-
-
- Where,
-
-
- L= LOQ of impurity in ppm
-
-
-
- C= Concentration of drug substances (theoretical) in ppm from Sample preparation-1
-
-
-
- A= Area of drug substances from Sample preparation-1
-
-
-
- RF= Response factor of impurity.
-
-
- In the case where RF is mentioned in the GTP corrected area of respective impurity shall be used in the calculation.
-
Chromatogram Checklist (Chromatography Review) : (Annexure 2)
-
- Fill the detail like Product / Sample, Test, Reference (GTP/Protocol No./Template No.), Bach No., A.R.No., in Chromatogram checklist.
-
- Put the “ √ ” mark against the applicable point and ‘NA’ against the not applicable points.
-
- If any parameter in the Chromatogram checklist is not complying or not carried out writing the justification under the head “Remark” or if the deviation is filled mention the deviation no.
-
- Make a bunch of “Chromatogram checklist”, “Mobile phase preparation “Sequence print out”, instrument method, processing method, and all chromatograms and attach with the template/worksheet or protocol.
-
- Put all chromatograms together and attach them with the relevant Batch No./ A.R.No. Document.
-
- In case of analysis discontinuation, mention the reason for discontinuation in instrument usage Log and on the “Chromatogram checklist”.
-
- Take the print out of all generated chromatograms put the canceled on each chromatogram with proper justification and attaches with the relevant document.
-
General Guideline and instructions for Chromatography:
-
- Chromatographic systems shall be reviewed by the reviewer for events, such as,
-
-
- Unprocessed chromatograms,
-
-
-
- Single injections,
-
-
-
- system check injections,
-
-
-
- system suitability injections,
-
-
-
- Partially run sequences or complete run sequences,
-
-
- Which are not part of the original sequences on a weekly basis and
-
- Document the review observations as per the current version of SOP for Analytical Data Review.
-
- Any of the above events are observed, log the lab incident as per the current version of the SOP-Lab Incident.
-
- Chromatographic run shall be identified, processed, justified and impact assessment shall be performed w.r.t. original sequence. Prints out shall be attached to the original sequence.
-
- For any chromatographic run incident, such as incomplete run, discontinued run or run for system suitability check (or for any other reason ) and not considered for evaluation shall be marked as “Not Used” with proper justification, signature, date, and shall be made part of main sample sequence set attached with the batch analytical documentation.
-
- “Printed on date and time” of chromatograms shall be part of the chromatogram report format.
-
- Chromatograms shall be processed within one working day from the completion of the sequence.
-
- QC head shall identify instruments that are not in compliance with 21 CFR part 11.
-
- Derive an action plan with a timeline to make all such instrument compliant (refer current version of SOP for Data Integrity).
-
-
For experimental analysis sample shall be selected from,
-
-
-
- Expired lots of finished products.
-
-
-
- Lots prepared from working standard.
-
-
-
- Retention Sample (subject to Approval of Head QA)
-
-
- All the activities performed by the service technician shall be recorded in the report and the same shall be reviewed and approved as per respective report approval procedure.
-
- For related substances test / Degradation product, New cleaned vial shall be used.
-
-
Run time and replicate injection.
- In the test for Assay, run all Standard and Sample chromatograms about 5 minutes extra after the principal peak elution is over and the peak is properly integrated or as per the GTP / STP.
-
-
- Chromatography Purity/ Degradation/ Related Substances/Stability samples analysis, run the chromatogram 2.5 times the RT of principal peak or as specified in individual GTP / STP.
-
- In case of specific impurity analysis, run the chromatogram about 5 minutes extra after the principal peak elution is over and the peak is properly integrated.
-
- In case the HPLC system is running in the mobile phase (subject to sufficient mobile phase volume available) and the further sample is supposed to inject,
-
-
It can be injected as per the following strategy.
-
-
-
- If the time gap is 2 hrs. or less further samples can be injected directly.
-
-
-
- If there are more than 2 hrs. time-gap inject bracketing standard.
-
-
- Calculate the RSD of bracketing standard (i.e. last two injections of initial system suitability standard and injections of bracketing standard made after time gap) and if this is satisfactory further sample analysis shall be continued.
-
- In the end of the sample sequence a different method for “D2 lamp off and flow rate change to 0.2ml/min (or suitable)” shall be submitted to keep the system stabilize in the mobile phase.
-
- If any carryover in the chromatogram is not affecting the interested peak analysis shall be considered after proper justification and authorization.
-
- The concentration of any unknown peak in assay, dissolution, and content uniformity test, shall not be more than the concentration of unknown impurity peak in related substances test. (As related substances unknown impurity limit is derived based on ICH daily dose criteria.)
-
-
Any unknown peak detected above this concentration shall be investigated.
-
-
- During analysis, an additional injection of blank-mobile phase and/or diluent can be made to verify the elution pattern of the blank.
-
- Additional injection of placebo solution and impurity solution with or without spiking can be made where ever necessary with proper justification.
-
- In case, if during analysis any failure in established system suitability, peak splitting, area variation shall be rectified on line by altering the same sequence with justification.
-
- In the case of the sample if any abnormality is observed during analysis then a freshly prepared sample can be injected with proper justification.
-
- If the system is required to be discontinued for rectification then it shall be handled as per SOP on Lab Incident.
-
-
Preparation and use of specimen chromatograms
-
-
- To verify the reproducibility in chromatography and
-
- To notice any change in chromatograms online during analysis and also
-
- At the verification stage during raw data review by comparing the chromatogram against specimen chromatograms, the laboratory shall maintain a file of “Specimen Chromatograms “for each HPLC test ( product wise).
-
- Reproducibility can also be verified by AMV data.
-
- To prepare the specimen chromatogram and ensure its usage the below procedure to be followed.
-
- If the reference chromatogram is available from the manufacturer, Source laboratory STP/ Data, the chromatogram of first analysis/ analytical method transfer (or method validation) shall be compared against the reference chromatogram to check the following but not limited to,
-
-
- Peak shape
-
-
-
- Retention time
-
-
-
- Baseline (Baseline pattern is very important particularly in HPLC gradient analysis)
-
-
-
- Scale of chromatogram
-
-
-
- Additional peak
-
-
-
- Other system suitability parameters as per the requirement of STP / ATP.
-
-
-
Make a photocopy of one chromatogram each of
-
-
-
- Blank (diluent),
-
-
-
- Resolution solution,
-
-
-
- Standard solution,
-
-
-
- Placebo solution,
-
-
-
- Sample solution, etc. as per the requirement of the test,
-
-
- Put “Specimen chromatogram” on individual chromatogram and sign/date of Head QC.
-
- File all the “ Specimen chromatograms” product and test wise for the comparison of all future analyses.
-
- During routine analysis, the analyst shall verify the chromatogram against Specimen Chromatograms.
-
- In case of any abnormality observed in the chromatographic pattern analyst shall immediately inform QC -Head or designee.
-
- QC-Head or designee shall suggest an analyst for allowable modification to get the chromatography that is comparable with the “ Specimen chromatogram”.
-
- During raw data review, the reviewer shall verify the chromatograms against the “specimen chromatogram”, in case of any abnormality observed.
-
- Whenever there is any change in method of analysis, the impact on the “Specimen chromatogram” shall be evaluated.
-
-
Cancellation of Chromatograms :
-
-
- Each cancellation of chromatograms due to system failure, system suitability parameters failure, poor chromatography or due to any other reason, shall be canceled by section head only.
-
- Section head shall put the canceled stamp on each canceled chromatograms with proper justification with sign and date.
-
HPLC change oversteps from reverse phase to Normal phase chromatography
- Step 1:
-
- After proper washing of the previously used column remove the column from the column compartment and connect the Union.
-
- Keep all tubing reservoirs in the bottle containing HPLC water/Solvent (degassed) and proceed following steps.
-
- Step 2: Dry Prime:
-
- Take 100 % HPLC water in Mobile phase reservoirs, and execute the dry prime 5 minutes individually for each channel (line) at flow rate 5ml/minutes during the dry prime outlet valve shall be opened.
-
- Step 3: Wet Prime:
-
- Execute the wet prime 3 minutes individually for each channel (line) at flow rate 5ml/minutes during the dry prime outlet valve shall be opened.
-
- Note: Degasser shall be off during priming (preferably in Quaternary pump)
-
- Step 4: Needle Wash, Seal Wash, Injector Wash:
-
- Perform these activities for the time as per default settings subsequently.
-
- Step 5:
-
- Flush the HPLC system for 20 minutes by gradually increase the flow 1ml/minute to 5ml/minute, 25% flow from each channel / Pump (line).
-
- Step 6 :
-
- Repeat wet prime, needle wash, seal wash, and injector wash as per procedure mentioned above in steps 2, 3, and 4 by using 100% HPLC grade methanol.
-
- Step 7 :
-
- Repeat wet prime, needle wash, seal wash, and injector wash as per procedure mentioned above in step 2, 3, and 4 by using 100 %HPLC grade IPA.
-
- Step 8 :
-
- Use 100% IPA or other non-polar solvents as per GTP or template for seal wash or needle wash during the analysis.
-
HPLC change oversteps from the Normal phase to Reverse phase chromatography.
- Step 1 :
-
- After proper washing of the previously used column remove the column from the column compartment and connect the Union.
-
- Keep all tubing reservoirs in the bottle containing HPLC water /solvent (degassed) and proceed following steps.
-
- Step 2: Dry Prime:
-
- Take 100 % Isopropyl Alcohol (IPA) in Mobile phase reservoirs, and execute the dry prime 5 minutes individually for each channel (line) at flow rate 5ml/minutes during the dry prime outlet valve shall be opened.
-
- Step 3: Wet Prime:
-
- Execute the wet prime 3 minutes individually for each channel (line) at flow rate 5ml/minutes during the dry prime outlet valve shall be opened.
-
- Note: Degasser shall be off during priming (preferably in Quaternary pump)
-
- Step 4: Needle Wash, Seal Wash, Injector Wash:
-
- Perform these activities for the time as per default settings subsequently.
-
- Step 5:
-
- Flush the HPLC system for 20 minutes by gradually increase the flow 1ml/minute to 5ml/minute, 25% flow from each channel (line).
-
- Step 6:
-
- Repeat wet prime, needle wash, seal wash, and injector wash as per procedure mentioned above in step 2, 3, and 4 by using 100% HPLC grade methanol.
-
- Step 7:
-
- Repeat wet prime, needle wash, seal wash, and injector wash as per procedure mentioned above in step 2, 3, and 4 by using 100 %HPLC grade water.
-
- Step 8:
-
- Flush the HPLC system for 20 minutes by gradually increase the flow rate 1 ml/min to 5 ml/minutes, 25% flow from each channel (line), (In case of the Quaternary pump).
5.0 Reference & Annexure – Good Chromatography Practices :
-
-
References :
-
-
- 21 CFR Part 11: Electronic Records ; Electronic Signatures
-
- Schedule L1: Drug and Cosmetic Act (Good Laboratory Practices)
-
- USP – NF: USP 40, NF -35, Chapter <621>
Annexure 1: Mobile Phase Preparation Record
| Product / Material : …………………………………………………………………………………………
B. No. / A.R. No. : 1. ………………….. 2. ………………..3. …………………. 4. ………………… Tests : 1. …………………. 2. …………………… 3. …………………….. 4. …………………… Mobile Phase Quantity: …………………………………ml Date of preparation: ………………………………… Prepared by: ………………………………… Date of Usage: ………………………………… Quantity Used: …………………………………. Quantity Destroyed: ………………………………… Destroyed by (Sign/Date): ………………………………… |
Annexure 2: Chromatogram Checklist
| Product / Sample: _______________ A.R. No.: ________________
Test :_____________________ Batch No.:_____________________
|
|||||||||||||||||||||||||||||||||||||||||||


