Standard Operating Procedure (SOP) to establish the hold time of drug samples at the different stages of manufacturing (like starting material/Raw material, Intermediate and bulk product) prior to the final packing of the drug.
Procedure to Establish the Hold Time of Samples
1.0 PURPOSE:
-
- The purpose of this SOP is to describe the systematic procedure to establish the hold time of starting materials, intermediates and bulk products prior to the final packing of drug products.
2.0 SCOPE:
-
- This SOP is applicable for raw materials, intermediate as well as bulk product sample hold time to study at the pharmaceuticals drug manufacturing plant.
3.0 REFERENCES:
-
- In House

- In House
-
- WHO Technical Report Series No. TRS-992, 2015
4.0 RESPONSIBILITY:
-
-
QA shall be responsible for-
-
-
- Collect the hold time in-process samples as per hold time study and shall send duly filled “Test Requisition Cum Report” to the QC department.
-
- Maintain hold time register and soft copy of hold days record (Attachment 2).
-
- Investigate in case of failure/discrepancies.
-
-
QC shall be responsible for-
-
-
- Make entry of the sample as per defined procedure and analyze the sample as per the defined procedure.
-
- Share the report and investigate in case of failure/discrepancies.
-
- Production shall be responsible to prepare the Test Requisition cum report and intimate to QA for sampling.
-
- Quality Head and Plant Head shall be responsible for review and approve the SOP.
5.0 ABBREVIATIONS:
-
- HDPE: High-density Polyethylene
-
- MPS: Master product specification
-
- NA: Not Applicable
-
- WHO: World Health Organisation
6.0 DEFINITION- SOP FOR HOLD TIME OF SAMPLES:
-
- Intermediate: Partly processed product that must undergo further manufacturing steps before it becomes a bulk product.
-
- Bulk Product: Any pharmaceutical product that has completed all processing stages up to, but not including final packaging.
7.0 PROCEDURE FOR HOLD TIME STUDY OF SAMPLES:
-
-
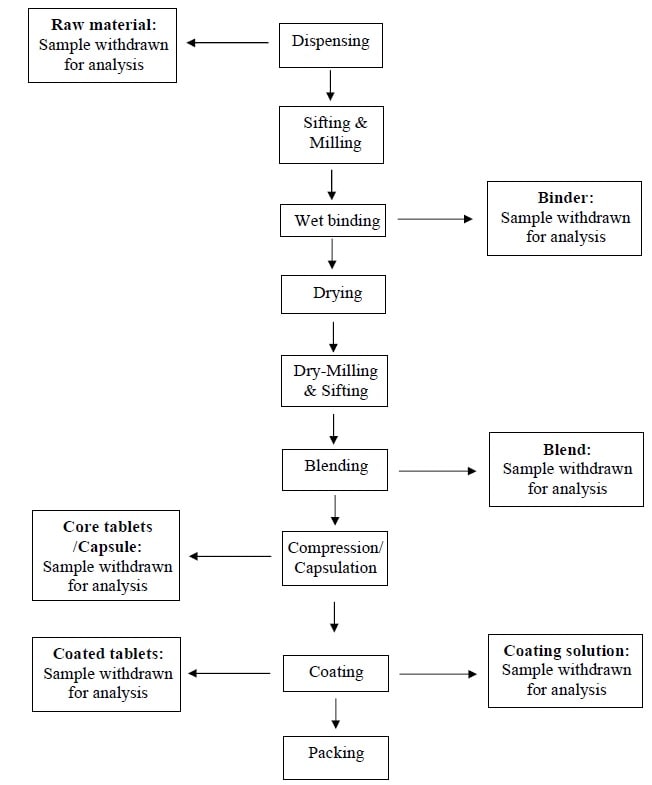
Process flow chart- Hold Time of Samples
-
-
- Perform the Hold Time Study of Samples in one batch, which shall represent the respective product.
-
- Perform the Hold Time Study of Samples as per customize the protocol.
-
- Prepare separate protocol and followed for different dosage forms and customize as per formulation.
-
- Each protocol shall cover hold time study of samples at different stages during manufacturing i.e.
-
-
- Raw materials (dispensing),
-
-
-
- Granulation fluid & granular blend (granulation),
-
-
-
- Core tablet (compression),
-
-
-
- Coating suspension & coated tablet (coating) and
-
-
-
- Filled capsule (Capsulation).
-
-
- Mention the storage condition of the product in the protocol as per dosage form for hold time study.
-
- Store the containers of hold time samples in the same pack (i.e. HDPE or S.S. container) as used in production.
-
-
Reducing the size of the container, when this is necessary for testing holding time, shall be justified.
-
-
- The environmental conditions for sample storage shall be the same as those of the quarantine area/manufacturing stage and mentioned in the respective protocol.
-
- Establish and define a sampling plan in the protocol for taking samples for testing at different intervals.
-
- Calculate the amount of sample required based on the interval, test required in respective product/material specification or analytical test procedure and mention the quantity in the respective protocol sampling plan.
| Stage | Test (customized as per the requirement) | Study time |
| Raw material | Description, Moisture content, Assay, Microbial test | Initial, 15th & 30th day |
| Granulating fluid & Coating suspension | Physical appearance, specific gravity, sedimentation, viscosity, pH, Microbial test | Initial, after 24 & 48 hours |
| Granular blend/Pellets | Description, Loss on drying, blend uniformity, particle size bulk/tapped density, Microbial test | Initial, 15th & 30th day |
| Core tablets-uncoated | Description, hardness, thickness, dissolution, related substances, uniformity of dosage unit, Assay, Microbial test | Initial, 15th & 30th day & up to 60th day if finished product is uncoated. |
| Coated tablets | Description, hardness, thickness, dissolution, related substances, uniformity of dosage unit, Assay, Microbial test | Initial, 15th, 30th & 60th day |
| Capsules | Description, locking length, dissolution, related substances, uniformity of dosage unit, Assay, Microbial test | Initial, 15th, 30th & 60th day |
-
-
Hold time study shall be performed in the following case:
-
-
- Storage condition change.
-
-
- Change in the formulation like addition or deletion of ingredients.
-
-
-
- Change in manufacturing process like change in granulation method etc.
-
-
-
- For new formulation
-
-
-
The hold time study for tablet and capsule shall be performed up to :
-
-
-
For blend, granulating fluid, coating suspension & tablet:
-
-
- Hold granulating fluid/coating suspension for 48 hours (samples send to QC at initial, end of 24 & 48 hours).
-
- Blend for 30 days (sample send to QC at Initial, 15th & 30th day)
-
- Hold core tablet for 60 days (sample send to QC at Initial, 30th & 60th days)
-
- Hold Coated tablets for 60 days (sample send to QC at Initial, end of 30 & 60 days)
-
-
For Capsules:
-
-
- Hold blend for 30 days (sample send to QC at Initial, end of 15 & 30 days)
-
- Hold filled capsules for 60 days ( sample send to QC at Initial, end of 30 & 60 days)
-
- In case of New Product, the Hold time period for tablet and capsule are given below:
-
- Blend should be compressed/filled within 15 days
-
- Core tablet should be coated/packed within 30 days from the date of compression.
-
- Coating suspension should be used before 24 hrs from the preparation of coating suspension
-
- Coated tablet should be packed within 30 days from the date of coating.
-
- Filled capsule should be packed within 30 days from the date of filling.
-
-
Hold time samples (Granulating fluid, Blends, Core tablets, Coated tablets & Coating Suspensions) handling during tablet manufacturing:
-
-
-
Raw Materials:
-
-
- QA person shall collect the required quantity of sample of starting materials as per the protocol and stored in the HDPE container in quarantine.
-
- The entry of hold time samples collected should be made in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
-
-
Granulating Fluid:
-
-
- QA person shall collect the required quantity of sample of granulating fluid (binder) as per the protocol and stored in the S.S. container in controlled area.
-
- The entry of hold time samples collected should be made in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
-
-
Blend Stage:
-
-
- QA person shall collect the required quantity of the sample of blend/granules/pellets as per the protocol or as specified in BMR/MPS and stored in the HDPE container in blend Quarantine.
-
- The entry of hold time samples collected should be made in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
-
-
Compression Stage:
-
-
- QA person shall collect the required quantity of the sample of core tablet as per the protocol and stored in the HDPE container in tablet Quarantine.
-
- The entry of hold time samples collected shall be made in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’ shall be filled by QA and send to QC along with the sample.
-
-
Coating Stage:
-
-
- QA person shall collect the required quantity of the sample of coated tablet as per the protocol and stored in the HDPE container in tablet Quarantine.
-
- The entry of hold time samples collected should be made in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’ should be filled by QA and send to QC along with the sample.
-
-
Coating Suspension:
-
-
- QA person shall collect the required quantity of coating suspension as per the protocol and stored in the S.S container in the tablet Quarantine.
-
- Make the entry of hold time samples collected in the hold time sampling register.
-
- On completion of hold time, QA shall fill the ‘Test Requisition Cum Report’ and send to QC along with the sample.
-
-
Hold time of samples (Blends, Capsules) handling during capsule manufacturing:
-
-
-
Blend Stage:
-
-
- Follow the procedure as described above
-
-
Capsule Filling Stage:
-
-
- QA person shall collect the required quantity of filled capsule as per the protocol and stored in the HDPE container in the tablet/capsule Quarantine.
-
- Make the entry of hold time samples collected in the hold time sampling register.
-
- On completion of hold time, ‘Test Requisition Cum Report’. Filled by QA and send to QC along with the sample.
-
- The raw materials, blend, uncoated tablets, coated tablets; Store capsules by wrapping in double polyethylene bag lined by cleaned High Density Polyethylene container.
-
- Store the intermediate and bulk product samples under controlled environmental condition.
-
- If the hold time period of the product is exceed from the predefined period then fill the deviation and give the proper justification.
-
-
Hold time (Tablets/capsule/sachets/bottle/pouch) handling at packing stage :
-
-
- For Tablets/capsule/sachets/bottle/pouch which kept in factory premises at specified storage condition beyond the hold time data specified, sample shall be send to QC for analysis before release of batch.
-
- Head QA/Quality head shall recommend the testing parameter for re-testing, Preferably stability indicating test shall be consider for retesting (Assay, Average weight, Fill weight, Related Substances (impurities) dissolution etc.).
-
- Packing officer shall prepare “Test requisition cum report” slip and forward to IPQA officer for collection of sample.
-
- For chemical testing 50 units for tablet/capsule and for bottle/sachets 50 gm or as per QC requirement.
-
- For microbiology testing required 10 gm. or as per microbial requirement from each dosages form separately shall be send to QC.
-
- Attach the Certificate of analysis of Hold time sample stage receive from QC department with respective BMR/BPR.
-
- Update the Hold time in soft copy for future reference as attachment-2.
-
-
Transfer the Batch only after ensuring the compliance of QC result.
-
-
- The retest result shall be considered valid for 30 days from the date of last release report from QC and batches shall be released if the hold time is less than the total hold time.
-
- E.g. Previous established hold time is 80 days,
-
- A current “XX” batch hold up to 110 day, then the releasing criteria will be 80 days +30 days(grace period)=110 days.
-
- The total hold time of “XX” is 109 days, which is less than net hold time allowed i.e.110 days, this can be released without any retesting,
-
- However the retesting can be critical further compression of obtained retest results with initial result to be done prior to batch release, if any objectionable result reported shall be reported to QA/Quality Head for decision.
-
- In case a batch /product in retested and result found the complying then the existing hold time shall be updated accordingly for the future reference.
8.0 ANNEXURES:
Annexure 1: Hold Time Study Sampling Register.
Prepare the register/logbook by using following table contents..
-
- Sr. No
-
- Date
-
- Product Name
-
- Batch No
-
- Stage
-
- Sampled Quantity
-
- Location
-
- Initial sample send to QC
-
- Sample submitted by
-
- IInd sample send to QC
-
- Sample submitted by
-
- IIIrd sample send to QC
-
- Sample submitted by
-
- IVth sample send to QC
-
- Sample submitted by
-
- Remarks
Annexure 2: Hold Time Study Data Logbook.
|
Sr. No. |
Product Name | Item Code | Finished Product Hold Days | Type
(strip/blister etc.) |
Effective Batch No./ Date |



Pingback: Quality Manual & Quality Policy in Pharmaceuticals - Guideline
Pingback: Drug Product (Finished, Stability) Sampling Procedure - Pharma Beginners