Standard Operating Procedure (SOP) for Process Standardization and Validation be carried for the new products and existing products at the formulation plant.
SOP for Process Standardization and Validation
1.0 Purpose:
-
- To lay down a procedure for process standardization and process validation study.
2.0 Scope – Process Standardization and Validation:
-
- The SOP is applicable to process standardization and validation to be carried for all the new products and existing products at the formulation plant.
3.0 Responsibility :

-
- Executive/Officer: Execution of validation activity
-
- Head Production, Head Quality Assurance: Review and Approval of the protocol.
4.0 Definition- Process Standardization and Validation:
-
-
Process Validation-
-
-
- Establish documented evidence which will provide a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specification”.
-
-
Validation Protocol:
-
-
- A written plan stating how validation will be conducted, including test parameters, product characteristics, production equipment, and decision points on what constitutes acceptable test results.
-
-
Critical Process Parameters (CPP)-
-
-
- A process parameter, e.g. temperature, time, speed, which can affect the critical quality attributes of a product.
5.0 Procedure – Process Standardization and Validation :
-
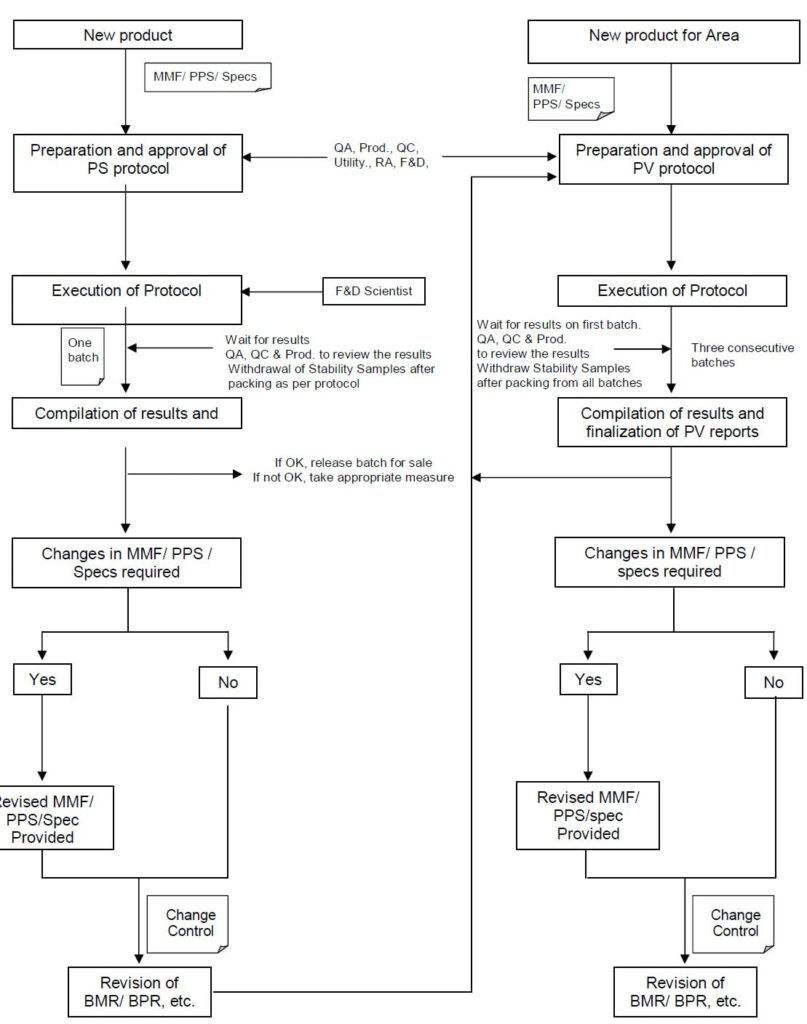
- One batch for process standardization based on MMF shall be taken for each product manufactured first time at manufacturing location to fix the critical process parameters, which can affect the critical quality attributes of the product.
-
- Three batches shall be taken for process validation for a product, which is under manufacturing at any of the manufacturing site or the product where standardization batch has been completed.
-
- Prepare the Process validation protocols on the generic name of the product.
-
Process Standardization (SOP for Process Standardization and Validation)
-
- Conduct Process standardization shall be based on a pre-approved protocol (Refer to Annexure-1).
-
- Conduct Process standardization on a product, which is never been manufactured at any site of the company.
-
- Prepare the sampling plan to cover the critical process parameters like blending time, drying temperature, compaction force, compression speed, coating process, packing, etc.
-
- Perform the manufacturing and sampling as per Batch Manufacturing/ packing records and Process Standardisation Protocol.
-
- Any deviation during standardization in the processor from specification shall report in the batch record.
-
- Provide the evaluation and justification for any atypical results or deviations.
-
Process Validation (SOP for Process Standardization and Validation)
-
- Process validation shall be based on a pre-approved protocol (Refer to Annexure-1).
-
- Take three batches for process validation for each existing product or the product where standardization completed.
-
- Prepare the Sampling plan, part of the Process Validation Protocol, to cover the critical process parameters like blending time, drying temperature, compaction force, compression speed, packing, etc.
-
- The batches shall be manufactured and sampling shall be performed as per Batch Manufacturing/ packing records and Process Validation Protocol.
-
- Report any deviation during validation in the processor from the specification on batch records.
-
- Provide evaluation and justification for any atypical results or deviations.
-
- After completion of the validation exercise, Evaluate and complile the results.
-
- In case there is no change in the batch documentation or specification, Use the same documents.
-
- Appropriately revise the document in case of any change.
-
- All changes shall be initiated, evaluated, and recorded through a change control procedure (SOP for Change Control Management).
-
- The addendum to Process Validation Protocol/ Report shall be taken for the cases where additional equipment needs to be included in the batch manufacturing/ packing.
-
- Define the validation and stability study requirements in respective change control.
-
Preparation of protocol cum report (SOP for Process Standardization and Validation)
-
- Protocol cum report (henceforth referred to as Protocol) shall be prepared for the initiation, recording, and control of Process Standardization/ validation activity.
-
- The protocol shall define manufacturing conditions, testing, and expected outcomes at each stage of process validation/ standardization.
-
- The format for protocol is attached as Annexure-1.
-
- For the preparation of protocol, a minimum of the following documents shall be required-
-
-
- Master Manufacturing formula
-
-
-
- Product Packing Specification/ Master Packing Card.
-
-
-
- QC Specification of the active ingredient, Blend/ Bulk specification, In process Specification & Finished products specification
-
-
-
- Batch manufacturing record.
-
-
- A protocol shall have a basic of below-mentioned parameters/ elements
-
-
- Provision of pre and post-approval of the protocol
-
-
-
- Reference to master documents like MMF, PPS, Specification, and test specifications/ methods used.
-
-
-
- The manufacturing conditions
-
-
-
- Scope of protocol wrt no. of batches and reason for carrying out the validation
-
-
-
- Starting material description including Analytical Reference Nos. and vendor details.
-
-
-
- The sampling plan includes sampling points, number of samples, and frequency of sampling.
-
-
-
- Diagrammatic reference wherever possible shall be provided.
-
-
-
- Data to be collected
-
-
-
- Tests/ Analysis to be performed
-
-
-
- Details of equipment/instruments used
-
-
- Statistical methods to be used for defining intra-batch and inter batch uniformity e.g. Relative standard deviation, Process capability index shall be used
-
Provision for recording data
- Provision for the recording of observation/ deviations / aberrant results or other information that has a bearing on successful completion of validation/ standardization and proposed corrective and preventive actions
-
- Summary, Conclusion, and Approval report that summarizes the process standardization/ validation outcome and indicates status validation.
-
- Heads of QA, QC, and Production shall approve the conclusion.
-
- Before the execution of the validation or standardization activity, the protocol shall be approved by various functional areas.
-
- The protocol shall be prepared by Quality Assurance.
-
- The Protocol shall be reviewed by the representative from QC, Utility, Production.
-
- The final approval shall be jointly approved by Heads of Production and QA.
-
- The protocol shall be approved by regulatory in case of products manufactured for the purpose of regulatory filling or manufactured exclusively for export.
-
Execution of protocol cum report
-
- The samples shall be collected as per the sampling plan defined in the protocol.
-
- The analysis of samples shall be as per the defined procedure mentioned in the protocol.
-
- During validation, samples shall be withdrawn in duplicate/triplicate.
-
- One set of samples shall be submitted to quality control for analysis.
-
- The other set shall be preserved in IPQA. In case of failure results, the second set of samples shall be used for investigation.
-
- During report preparation, the data generated shall be thoroughly reviewed with respect to acceptance criteria and designed parameters.
-
- The result of various parameters, deviations observed during manufacturing shall be reviewed by QA and Production or/and QC for evaluation.
-
- Corrective and preventative actions (CAPA) shall be suggested based on the results of the evaluation.
-
- R&D shall be duly informed of the same and necessary revision to Specification, Standard Test Procedures, Master Manufacturing Formula, and PPS.
-
- Necessary changes, if any as recommended in the report, after review in the process/parameters shall be done following the change control procedure.
-
- If however, there are no changes in any of the steps of parameters thereby not promoting the revision of batch records, the same version of batch records may be used for further manufacturing.
-
- After completion of process validation, a summary of validation shall be made as a part of the report stating various parameters/ ranges established during validation.
-
- Following the completion of the validation exercise, the report shall be concluded with the certificate of analysis. Heads of QC, Production, and QA shall approve the report by signing.
-
Stability Study
-
- All the standardization and validation batches shall be subjected for accelerated, intermediate, and long-term stability studies or based upon related change control. (Related: SOP for Stability Study of Drug Product)
6.0 Abbreviation – (SOP for Process Standardization and Validation)
-
- MMF: Master Manufacturing Formula
-
- PPS: Product Packing Specification
7.0 Annexures :
Annexure – I: Guidelines for the preparation of process standardization and validation Protocol cum report.
Sr. No. |
Guideline – Process Standardization and Validation (Part – A) |
|
1 |
After receiving a new MMF/ new formulation, Product packaging specification, Specification of API, Blend Specification, In-process specification & Finished product specification, QA personnel shall prepare standardization/validation protocol cum report and new BMR/BPR. |
|
2 |
Give the information like protocol cum report number, introduction, objective, and scope. The protocol shall be prepared in the generic name of the product. |
|
3 |
The standardization/validation protocol cum report shall be reviewed by Production, QC, F&D, RA, Utility, and finally approved by Head-Production & Head -QA. |
|
4 |
Details of responsibility of various departments in protocol preparation and approval shall be mentioned. |
|
5 |
Make necessary corrections as suggested by Production / QC / F&D / RA / utility after evaluating the standardization/validation protocol. |
|
6 |
Give a list of equipment, identification number of the equipment, Calibration date, Calibration due date (If applicable) which are required for the manufacturing of the product. |
|
7 |
Give a list of raw materials (components), its specification, Grade/ Brand name/item codes, approved source, and quantity used in the manufacturing. The details should be as per MMF. |
|
8 |
Draw process flow diagram indicating various steps involved in the manufacturing process, Equipment used, and critical steps involved in the manufacturing process of the product. |
|
9 |
Explain process steps – Blending/ mixing, compression/filling, coating, packing, vial washing, and depyrogenation, etc. |
|
10 |
Draw the sampling location diagram in case of blending/mixing to explain the location of sampling points. Draw the sampling location diagram for withdrawing the Samples during/after drying. The sample quantity at each location shall be drawn as per protocol. Clearly define the time spent or the minimum quantity of unit packs like a strip/ a blister/ or a container/vial or a pouch which shall be subjected under/for validation |
Sr. No. |
Guideline – Process Standardization and Validation (Part – B) |
|
1 |
At dry mix where active ingredients load is less than 50% w/w shall be subjected to Blend uniformity test. |
|
2 |
Give details of punch specifications, compression parameters – such as Uniformity of weight, average weight, group weight, thickness, hardness, friability, disintegration time, etc, Capsule filling parameters such as Uniformity of weight, Average weight, group weight, Lock length. |
|
3 |
Give the study, test requirement, and sampling details of the Tablet compression machine at maximum and minimum hardness, Maximum speed and minimum speed, and at various stages of compression viz., initial, middle, and end-stage at the optimum hardness and optimum speed. |
|
4 |
Give the study and sampling details of the capsules filling machine at maximum speed and minimum speed and at various stages of filling viz., initial, middle, and end-stage. |
|
5 |
The sampling details & test requirements may change as per the recommendation by the R&D. |
|
6 |
Mention coating parameters – Atomizing pressure, Bed temperature, Inlet air temperature, Outlet temperature, Coating pan RPM, peristaltic pump speed, Distance of gun from the bed, etc in case of tablets coating. |
|
7 |
Give the study and sampling details of the tablet-packing machine (Blister & strip) at high sealing temp with slow speed, low sealing temp with high speed and optimum temp, and optimum speed at initial, middle, and end-stage. During induction sealing, different height & different speeds shall be mentioned for the validation. |
|
8 |
Mention details of the sample quantities to be taken at different stages of the operation. |
|
9 |
Give acceptance criteria for blended material, compressed tablets, coated tablets, filled capsules, filled bottles/ vials, and packed tablets/capsules/ Bottles/ Vials as mentioned in BMR/BPR and product specification. |
|
10 |
At the time of manufacturing, process standardization/validation protocol cum report is given to IPQA and production for execution. |
Sr. No. |
Guideline – Process Standardization and Validation (Part – C) |
|
1 |
The sampling is carried out as per the predefined sampling plan given in the standardization/ validation protocol. |
|
2 |
Wait for results from QC for important stages like dry mixing, blending, pre-lubrication/ lubrication for Ist batch. |
|
3 |
After ensuring satisfactory results, the process shall proceed for the next step. |
|
4 |
Deviations in the process may be carried out. The deviation during the manufacturing & packing shall be mentioned in the process deviation sheet in the batch record. |
|
5 |
All the data for process standardization/validation protocol shall be compiled by QA. |
|
6 |
All deviations and summary reports in the standardization/validation protocol shall be made by QA. |
|
7 |
The final standardization/ validation protocol cum report shall be approved by Head-QA.
Note 1) If required any change in standardization/ validation, a changed version of MMF incorporating standardized parameters for the next batch shall be taken from F&D before starting a new batch. 2) After receiving the new version of MMF, QA shall prepare BMR by incorporating the standardized parameters for 3 validation batches. |
|
8 |
The process validation protocol shall be prepared and reviewed based on standardization batches. |
|
9 |
At the end of the execution of three batches, BMR shall be reviewed by Production and QA and approved by QA Head. |
|
10 |
All the standardization/validation batches shall be released for dispatch after approval of QA Head. |
Annexure-II: Process flow diagram for Process standardization/ Validation.