Standard Operating Procedure for Operation and calibration of UV cabinet for reviewing the TLC plate, Photolytic Degradation, UV cabinet Performance check.
SOP for UV Cabinet (Operation & Calibration)
1.0 PURPOSE:

-
- The purpose of this Standard Operating Procedure ( SOP ) is to describe the procedure for suitability of the emission intensity of the UV radiation used for the visualization of the spots in thin-layer chromatograms and for the photolytic degradation of the substance by UV light.
2.0 SCOPE:
-
- This procedure is applicable to the following UV cabinet at the quality control department.
3.0 REFERENCES:
-
- SOP for Handling of Out of Calibration (OOC)
-
- Instrument User Manual
-
- SOP for Maintenance of Laboratory Instruments
-
- SOP for Preparation of internal and external (Third Party) Calibration schedule and calibration practices.
4.0 RESPONSIBILITY:
-
-
Analyst shall be responsible for:
-
-
- Operation of the instrument as per SOP.
-
- Calibration of the instrument as per SOP.
Also read: SOP for Operation and Calibration of Analytical Balance
-
- To maintain the instrument usage log, calibration record, and history card..
-
-
Quality Control Head or Designee shall be responsible for:
-
-
- Approval of the calibration of instrument verifying against SOP.
-
- To give training to all the concerned persons before implementing the SOP.
-
- Execute the Out of calibration in case of calibration failure and in case of breakdown intimate to the Quality Head.
-
- Ensure the operation and calibration of the instrument is carried out as per SOP.
-
- Ensure proper documentation as per SOP.
-
-
Quality Assurance shall be responsible for:
-
-
- Ensure the implementation of the system as per the SOP.
Also read: Calibration of UV Spectrophotometer
-
-
Site Quality Head and QA Head shall be responsible for :
-
-
- Approval of the SOP.
-
- Implementation of the system as per SOP.
5.0 ABBREVIATIONS USED IN SOP FOR UV CABINET:
-
- ATP: Analytical Testing procedure.
-
- NIST: National Institute of Standard and Technology.
-
- USP:United States Pharmacopoeia.
-
- UV : Ultraviolet.
6.0 DEFINITION:
-
- NA
7.0 PROCEDURE FOR OPERATION AND CALIBRATION OF UV CABINET:
-
-
General Procedure :
-
-
- Follow the SOP on Instrument/Equipment usage logbook, for the entry of usage of the instrument.
-
- In case of any maintenance of the instrument, follow SOP on Maintenance of Laboratory Instrument,
-
- Maintain the third party calibration schedule and the Internal calibration schedule for the instrument as per SOP, Preparation of internal and external (Third Party) Calibration schedule and calibration practices.
Also read: HPLC Calibration- A Complete Guide
-
-
Operational Procedure of UV Cabinet for reviewing the TLC plate :
-
-
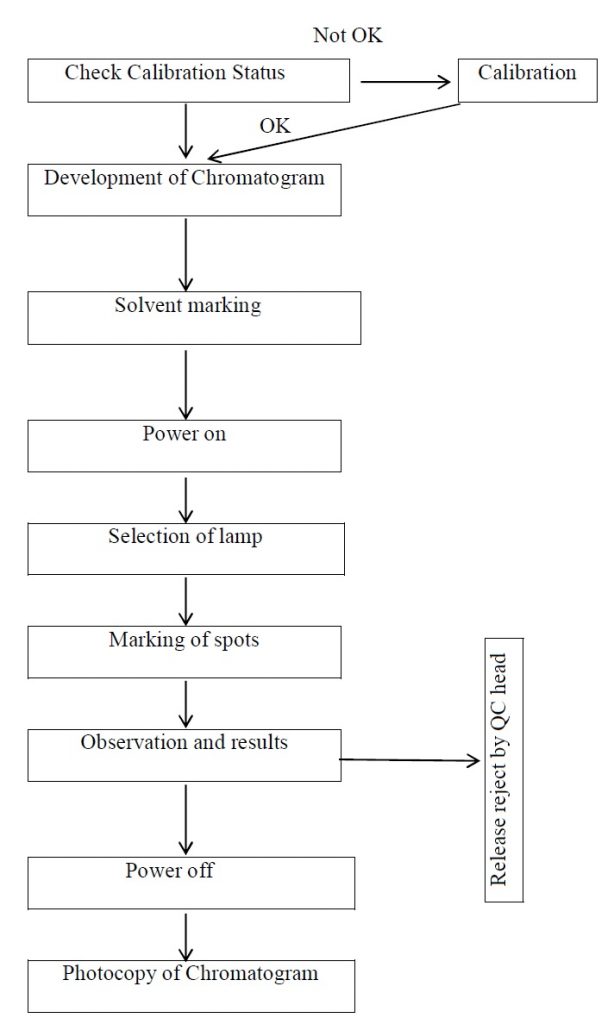
- Check the calibration status.
-
- Make the entry in the instrument usage log.
-
- Develop the chromatogram as per the procedure specified in the individual monograph.
-
- Mark the solvent front.
-
- Dry the plate in the air or as per the individual monograph.
-
- Treat the developed plate if specified in the monograph.
Also read: HPLC Calibration- A Complete Guide
-
- Check the UV cabinet for any unwanted things and remove them. Place the plate before switching ON the lamp in the cabinet. Close door of the cabinet.
-
- Switch on the mains.
-
- Select the lamp of specified wavelength (emission) by pressing the switch (red in color) towards the wavelength indicated on the board.
-
- View through the glass provided on the top of the cabinet.
-
- Mark the spots immediately.
-
- Switch off the power.
-
- Remove the plate.
-
- Calculate the Rf value by the formula,

-
- Make a photocopy of the plate on a paper with product details
-
- Attach the same to the report.
-
-
Operational Procedure of UV Cabinet for photolytic degradation :
-
-
- Check the calibration status.
-
- Make the entry in the instrument usage log.
-
- Open the door of the cabinet.
-
- Check the UV cabinet for any unwanted things and remove them.
Also read: SOP for Operation and Calibration of Analytical Balance
-
- Place the substance/product in a suitable for which the photolytic degradation is required for the time
- specified in the individual ATP.
-
- Switch on the mains.
-
- Select the lamp of specified wavelength (emission) by pressing the switch towards the wavelength
- indicated on the board.
-
- After specified time switch off the lamp and remove the container.
-
- Switch off the power.
-
- Treat the sample further as specified in ATP.
-
Calibration Procedure of UV Cabinet :
-
Performance Check of UV Cabinet for short-wavelength ( 254 nm):
- The analyst shall be prepared the 0.04% Sodium salicylate solution to check the performance for short wavelength(254nm)
-
-
-
04% Sodium salicylate solution preparation:
-
-
- Weigh accurately about 0.04gm of Sodium salicylate USP reference standard into 100 ml volumetric flask and dissolve it in ethanol (95 percent v/v) and dilute up to the mark with ethanol(95 percent v/v).
-
- The analyst shall apply a 5 mm spot of 5mL of 0.04% w/v of Sodium salicylate USP reference standard solution on silica gel plate GF254.
-
- The analyst shall immediately observe the spot into Ultraviolet cabinet using short-wavelength 254nm.
-
- Performance check shall comply at 254nm if the spot of 0.04% w/v of Sodium Salicylate USP reference standard (NIST Traceable) solution is clearly visible.
-
-
Performance Check of UV Cabinet for long-wavelength 365 nm:
-
-
- The analyst shall be prepared a 0.2% Sodium salicylate solution to check the performance for a long wavelength (365nm).
-
-
2% Sodium salicylate solution preparation:
-
-
- Analyst shall weigh accurately about 0.2gm of Sodium Salicylate USP reference standard into 100 ml volumetric flask and dissolve it in (95 percent v/v) and dilute up to the mark with ethanol(95 percent v/v)
-
- The analyst shall apply a 5mm spot of 5ml of 0.2% w/v of Sodium Salicylate USP reference standard solution on silica gel plate GF254.
Also read: SOP for Disintegration Apparatus (DT)
-
- The analyst shall immediately observe the spot into ultra Violet cabinet using long-wavelength 365nm.
-
- Performance check shall comply at 365nm if the spot of 0.2% w/v of Sodium salicylate USP reference standard solution is clearly visible.
-
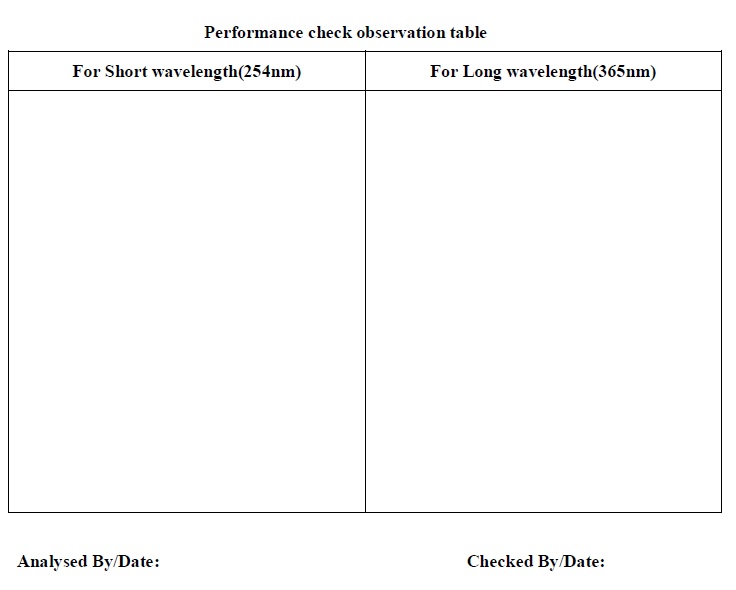
- The analyst shall draw the spot as per visualization on the performance check observation table given in Annexure-1.
-
- In Case the spot is not clearly visible replace the tube light and repeat the above performance check procedure.
-
- After TLC plate visualization is over, remove the TLC plate from the violet cabinet and switch off the ultraviolet cabinet.
-
- Calibration Frequency of UV Cabinet: Quarterly ± 7 days.
8.0 ANNEXURES:
-
-
Annexure-1: Calibration record of UV Cabinet.
-
| Instrument Name | UV Cabinet | ||
| Instrument Code | Make/Model | ||
| Location | Calibration Frequency | Quarterly ± 7 days | |
| Calibration Done on | Calibration due on
|
||
| Reagent/Solvent used | Batch No./Calibration Standard No. | Make/Grade |
| Sodium Salicylate | ||
| Ethanol |
TLC Spotter_____________________ Calibrated Valid Up to __________________________
-
-
Performance Check for long wavelength(365nm)
- Procedure:
-
-
- Select a pre-coated thin layer chromatographic plate with silica gel G as a coating material.
-
- Prepare a solution of Sodium salicylate in ethanol (95%)having a concentration of 0.2 % w/v by dissolving ______ 40mg of _____________________ Sodium salicylate in _____ 25 ml of _______________ ethanol (95 %). (Solution-A)
-
- Apply ______ 5mL of this solution (Solution A:0.2% w/v solution of Sodium Salicylate) on the plate.
-
- Immediately observe the spot into the Ultraviolet cabinet using long-wavelength 365nm.
-
- Check for compliance.
-
- Performance check shall comply at 365nm if the spot of 0.2%w/v of Sodium salicylate standard solution is clearly visible.
-
- Draw the spot as per visualization on the performance check observation table given in Annexure-5.
-
- Observation:______________________________________________;
-
- Conclusion: The Spot is observed /not observed clearly.
-
-
Performance Check for Short wavelength(254nm):
-
-
- Procedure:
-
- Select a pre-coated thin layer Chromatography plate with silica gel G as the coating material.
-
- Prepare a solution of Sodium salicylate in ethanol having a concentration of 0.04% w/v ,Pipette out 5 ml of __________solution-A(0.2% w/v Solution of Sodium salicylate)to _____25ml and dilute up to the mark with_________ethanol.
-
- Apply a 5mm spot of _____5µl of this solution (0.04% w/v solution of Sodium Salicylate)on the silica gel plate GF254.
-
- Immediately observe the spot into the Ultraviolet cabinet using short-wavelength 254nm.
-
- Check for compliance.
- Performance check shall comply at 254nm if the spot of 0.04% w/v of Sodium salicylate standard solution is clearly visible.
-
- Remarks: _____________________________________________________;
-
- The instrument is calibrated & qualified/Out of calibration & not qualified for use.
| Calibration : Schedule/Not Scheduled(Reason:__________________________________) |
| Calibrated By :
Date : |
Checked By :
Date : |
Approved By :
Date : |
-
-
Annexure-2: Format of the photocopy of the TLC plate.
-
-
- Paste here the Photocopy of TLC Plate with the following details…
| Name of Material | |||
| Batch No. | A.R. No. | ||
| Stage | Date | ||
-
-
Annexure-3: Flow chart for reviewing the TLC plate.
-
-
-
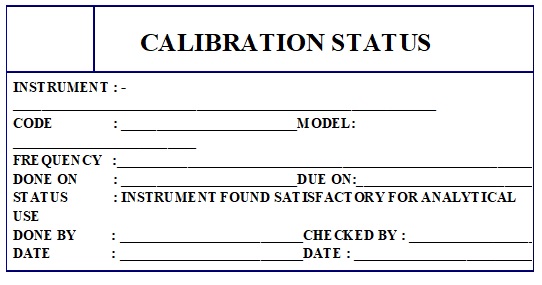
Annexure-4: Calibration Status Label.
-
-
-
Annexure-5: Performance check observation table.
-



Pingback: Operation and Calibration of pH Meter - Pharma Beginners
Pingback: SOP for Instrument Calibration (Internal & Third Party) - Pharma Beginners