Standard Operating procedure for receipt and storage of raw material. Starting material such as API and excipient required in the manufacturing of drug product.
Receipt and Storage of Raw Material
1.0 PURPOSE:
-
- The purpose of this SOP is to define the procedure for receipt and storage of raw materials used in various products.
2.0 SCOPE:
-
- This procedure is applicable to the receipt and storage of raw materials at the raw material store in the pharmaceutical manufacturing plant.
3.0 REFERENCES:
-
- Assigning expiry date to Excipients (SOP)
-
- Usage of API in case of API retest date exceeds retest date provided by the manufacturer (SOP)
-
- Redressing of Raw and Packing Materials (SOP)
-
- Guideline on Approval Rejection of material through ERP System
4.0 RESPONSIBILITIES:
-
- Warehouse Officer or Designee –
-
-
- Responsible for following the procedure of receipt and storage of Raw Material and maintain records.
-
-
- Warehouse Head or Designee –
-
-
- Responsible to ensure proper implementation of SOP.
-
-
- Quality Control Dept.
-
-
- Responsible to analyse and approve materials through Metis System.
-
-
-
- Responsible to intimate the Quality Assurance department in case of materials is not complying during the analysis as per the specification limit.
-
-
- QA Dept. –
-
-
- Responsible to ensure proper handling (receipt and storage) of material and take corrective measure, if any discrepancies during the analysis of raw materials.
-
-
- Quality Head and Plant Head –
-
-
- Responsible for approving the SOP.
-
5.0 ABBREVIATIONS:
-
- API : Active Pharmaceutical Ingredients
- CC No : Change Control Number
- COA : Certificate of Analysis
- CQ : Corporate Quality
- ERP : Enterprise Resource Planning
- GIM : Goods Inward Memo
- LR : Lorry Receipt
- NA : Not Applicable.
- PO : Purchase Order
- QA : Quality Assurance
- QC : Quality Control
- RM : Raw Material
- SOP : Standard Operating Procedure
6.0 PROCEDURE FOR HANDLING OF RAW MATERIALS:
-
Raw Material Receipt Procedure:
- During the receipt of raw material, security person shall inform to warehouse representative, and forward the documents to the warehouse.
-
- Warehouse representative shall check all the documents i.e.
-
-
- Purchase Order (PO)
- LR Copy,
- Cenvat Copy,
- MSDS,
- COA and
- Waybill / Road Permit, etc.
-
-
- After checking the documents, if found satisfactory, then return the documents to security for entry in the inward register.
-
- After inward entry, security person shall allow the vehicle in receiving bay for unloading of materials.
-
- If any discrepancies found, after correction or decide based on types of discrepancies, record the discrepancies detail in the backside of anyone documents of (P.O., LR Copy, Cenvat Copy, MSDS and COA etc.) & inform to concern for correction, and allow the vehicle for unloading the materials.
-
- Make correction prior to GIM preparation.
-
- After the arrival of the vehicle in the receiving bay, ensure necessary precaution like safety for unloading of materials.
-
- Check that material are protected and prevent to exposure of environment during transportation.
-
- Take necessary measures or protection while unloading the material during a rainy day.
-
Warehouse Checks:
- Check the following details before unloading the materials.
-
-
Documents Checks:
 Check security inward stamp on the backside of delivery challan/invoice after verification with Purchase order/address.
Check security inward stamp on the backside of delivery challan/invoice after verification with Purchase order/address.
-
-
- Excise documents accompany the material, in case the materials are excisable.
-
- Check and ensure the availability of vendor COA of the materials.
-
- In case of any document is not available to inform to supplier for further action and decide whether to receive the materials or not.
-
- Incase material can be receipt without available of certain documents then inform to concern, and document the details about the non-available of documents in the material documents (i.e. P.O., LR Copy, Cenvat Copy, MSDS and COA etc.) for further reference.
-
- Attache the said documents with original after receipt of the same.
-
- In case of items directly imported from the abroad manufacturer, additional documents of customs clearance like the bill of entry, invoice copy, manufacturer COA, excise bond etc. are required.
-
-
Vehicle Checks:
- Carry out the Proper vehicle inspection by Warehouse personnel and look for the following points.
-
-
- No sign of contamination such as petroleum distillate, corrosion or any type of
-
- No evidence of activity by insects, rodents or birds.
-
- Packages are properly stacked, and no sign of damage /broken /exposed /wet /leakage etc. are found.
-
- Check the intactness and seal of the materials bags/containers etc.
-
Acceptance criteria for raw material:
- Age of material at the time of receipt should not be more than 12 months old from the date of manufacturing.
-
- If any material having deviation from the criteria (Section 7.3.1), warehouse officer shall record the material details in (Annexure-5) and take the approval of QA Head for the authorization of materials.
-
- QA Head shall take the decision based on potent of the drug, shelf life, types of release, vendors etc.
-
- In case materials are received from other location of the same group of companies, accept the same and check the following.
-
-
- Intactness and proper labelling of container/bags. Loose bags having proper details with the label.
-
-
-
- No air entrapment in the bag.
-
-
-
- In case of material received from the same company Formulation location, first receipt the COA of that material, QC personnel shall review it, and if it complies with the entire specifications limit, then the material shall be received on the basis of requirement.
-
-
-
If COA is not complying with the specification limit, then materials shall not be received.
-
-
- If the material is under the retest period, then Concern formulation location shall analyze the material and after release, Provide its COA to site.
-
- COA complies all its specifications limit and with due consent of the Quality Head, the material shall be received as per requirement.
-
- Whenever expiry date and retest/ re-evaluation date of excipient is not available, expiry date shall be assigned as per below table.
-
- The same strategy for assigning shelf life to the excipient shall be followed at the time of new item code (Item master) generation in ERP.
| Sr. No. | Type of material | Expiry date from the date of manufacturing |
| 1 | Liquid Excipients | 36 Months |
| 2 | Solid Excipients | 60 Months |
| 3 | Solvents | 36 Months |
| 4 | Capsule | 24 Months |
| 5 | Colours (Lake & Soluble) | 36 Months |
| 6 | Flavours (Dry and Liquid) | 12 Months |
-
-
Note: The expiry date and retesting schedule of above-mentioned excipients can be reduced based on the nature of the material and/or any historical data.
- For active material, if the expiry date is not available on containers/bag/COA then warehouse shall get the expiry date from the manufacturer with the help of purchaser.
-
-
- Incase still expiry date is not provided by vendor then warehouse shall not receive the material, and material shall be under hold till the availability of expiry date or decision taken by management.
-
- If expiry of the material is not available, however, the retest/re-evaluation date of API is available then consider retest/re-evaluation date as the expiry date of the materials.
-
- For materials whose retest/re-evaluation date is considered as the expiry date of the material, the available stock (if any) of the same material can be used after re-analysis.
-
- The re-analysis of materials (expiry date not available) can be done for 4 instances.
-
- Use the Materials after ensures the Q.C. result and based on data revised expiry date shall be updated in Metis by QA.
-
- Beyond one year from the initial retest/re-evaluation date, Do not use the material in any product.
-
Raw materials unloading procedure:
- Follow the given below procedure.
-
- Open the Door/Shutter of material receiving bay and ensure that air curtain is “ON”, hoist door is closed.
-
- Unload the materials on clean pallets in the receiving bay by unloading persons.
-
- The unloading persons are not allowed to cross the black line and not to enter in the dedusting area.
-
- Same shall be followed by warehouse personnel, not to cross the black line & will not enter in the receiving bay during receiving of unloaded material.
-
- The unloaded material pallets shall betake inside the dedusting area by warehouse personnel without crossing the black line.
-
- Clean the containers/bags in the dedusting area either by using a vacuum cleaner or by a cleaned dry cloth.
-
- Ensure that containers/bags of material received are intact by checking the integrity of supplier’s seal, that’s not in damaged condition, and no other physically noticeable abnormalities are observed.
-
- In case materials are found short, damaged or any other physically noticeable abnormalities are observed, take the sign of transporters on delivery challan or transporters docket, and inform commercial department for information and necessary action.
-
- Keep (short, damaged or any other physically noticeable abnormalities) container on separate pallet & intimate QA/QC department for further action.
-
- Record short or damaged details in short/damaged material logbook i.e. (Annexure-4).
-
- If bags/containers are found in damaged condition, redress the material as per the SOP of “Redressing of Raw and Packing Materials”.
-
Inspection of Raw materials after unloading:
- After unloading of raw materials checks the following points.
-
- Check the item mentioned in the delivery challan/invoice against the item received.
-
- Check the manufacturer’s mother labels are affixed on all the container/bag.
-
- If any container/bag are found without label intimate to QA dept. for further action.
-
- Check the material has received from an approved vendor, if the vendor is not approved, then intimate to QA dept. for vendor approval in ERP system.
-
- Check the quantity of material received against the quantity indicated in the supplier’s delivery challan/Invoice.
-
- Verify the batch number, manufacturing date & expiry date mentioned if any on drum/bags against the mentioned in manufacturer COA.
-
- In case the batch number/ manufacturing date/ expiry date on container/bag is different from manufacturer COA then inform the commercial department and QC/QA for information and necessary action, and store the consignment in Quarantine area by affixing “HOLD” label (Annexure 2).
-
-
Perform the weight verification of all the container/bag on the basis of given below criteria
-
-
-
- In case, the number of received container/bag is 10 or less than 10, then weight verification shall be done of all container/Bag.
-
-
-
- If the number of received container/bag is more than 10, then weight verification of first 10 containers shall be done 100% and remaining container shall be weighed as per formula √n+1(Annexure 7).
-
-
-
- For Example Number of the received container is 15 then weighing of first 10 containers is 100% and for remaining (15-10=5) 5 containers, as per formula (√5+1=2+1) 3 container shall be weighed.
-
-
-
- In case of solvents: Weight of the drum shall be checked before dispensing, and if discrepancies intimate to concern person and record the details in Short/Damaged Material Logbook (Annexure 4).
-
-
-
- After weight verification write down the location code with suffix ‘Q’ (where material has stored) and prepare the receipt cum inspection report Refer (Annexure-3).
-
-
- If the material is excise exempted, put ‘X’ before suffix ‘Q’ of locator code or ‘EXEXQ’.
-
GIM Preparation of Raw Materials:
- On receipt of the material, Warehouse personnel shall check the material with its delivery challan/invoice.
-
- After checking the material Warehouse personnel shall do the physical verification and fill the details in Receipt cum inspection report (Annexure-3)
-
- During physical verification, if material found short from consignment, inform to warehouse Head/designee and QA Head. GIM shall be also prepared as per receipt short quantity.
-
- On the basis of Receipt cum inspection report and Delivery challan/LR, invoice, Warehouse personnel shall prepare the GIM (Unconfirmed) in Metis as per Location Code.
-
- Warehouse personnel shall take the printout of unconfirmed GIM (Annexure 6) and checked it against receipt cum inspection report, COA and delivery challan/invoice, then it shall do confirmed and verify by Warehouse Head/designee in Metis.
-
- Warehouse personnel shall send the printout of confirming GIM in QC with COA (Received from the manufacturer) for Analysis.
-
- After preparation of GIM, Warehouse personnel take the printout of Quarantine label and affix on the material container.
-
- In case, some container/bag/ corrugated box comes in damaged condition GIM / Distribution receipt to be made of full consignment and purchase return/distribution issue to be made (ERP system generated) of the damaged container.
-
- Deface the manufacturer’s approved labels and for other location Deface both (Approved & Quarantine) labels by crossing through permanent marker pen.
-
- In case, Goods inward memo preparation (GIM) of above consignment is pending due to any reason like non- receipt of proper excise document, manufacturer certificate of analysis, purchase order or ERP server Failure then Warehouse officer will store such type of consignment to the Quarantine area with status as “HOLD” as per (Annexure-2).
-
Labelling Procedure on Raw Material Container:
- Store officer shall generate Quarantine Label through metis system.
-
- Quarantine label affix after proper segregation of material.
-
- Affix quarantine label beside of supplier label.
-
- Affix the Quarantine labels (yellow coloured) on each container/bag of raw material (Annexure-1).
-
- If any extra label is required due to any reason, reprinting of the same label shall be done through the right of HOD only.
-
Sampling Procedure of Raw materials:
- Warehouse officer shall forward the GIM to QC department for sampling and analysis of materials.
-
- QC department shall sample and analyze the materials after receipt of GIM as per their plan.
-
- At the time of the release of material, QC shall remove suffix ‘Q’ and shall approve in Metis.
-
- If the material gets rejected, QC shall update the locator code as “REJ” in Metis and affix the rejected label on the material, as per the SOP of “Approval Rejection of material through ERP System”.
- Rejected material shall be transferred to the rejected area and after approval, it shall be disposed off.
-
- In case of any non-compliance subsequent to QC approval, Warehouse shall intimate QA department for further action, on the basis of investigation, QA will affix the “HOLD” Label (Annexure-2) and will make ‘Hold’ entries in the ERP.
-
Storage of Raw Materials :
- Checks and Precautions:
-
- Ensure that the containers are properly closed and are stored with intended containers.
-
- Store all the raw materials to their respective location.
-
- Ensure that clean pallets/ racks are available for stacking of materials.
-
- All the materials shall be stored only on racks/pallets, and no materials shall be kept on the floor.
-
- Store all the material in proper rows for easy movement of pallet trolley.
-
- In case of appropriate Quarantine, space is not available to store the raw materials, then the material can be stored in other areas by identifying and tied with yellow rope where temperature and relative humidity is maintained as per the specification.
-
- During storage separate materials with separate A.R. No. preferably store on separate pallets however in case of no availability of space/racks/pallets.
-
- Store all the raw materials in a manner to prevent the mix-up of materials by using separator/rope/shrink wrap in the racks/Pallets.
-
- Heavy containers preferable store at a low height and store the lighter container at and after 2nd
-
- Store solvents in the solvent storage area.
-
Storage Condition of Raw Material:
- Store all the raw materials in the area with respect to their storage conditions as per the storage condition list provided by the QA Department.
-
- QA shall ensure that the storage condition by referring vendors documents, manufacturing instructions on container labels, pharmacopoeia, MSDS and will mention storage condition regarding the same in storage condition list.
-
- Storage condition with respect to the area as per below table.
| Sr. No. | Temperature Range | Recommended storage condition |
| 1 | 2.0°C – 8.0°C | Refrigerated Cold Store |
| 2 | 25°C±2°C | AC room |
| 3 | Ambient Temperature | Only for monitoring |
-
-
Quarantine area:
-
-
- The material shows Suffix ‘Q’ affixed to respective location and yellow rope used as an additional identification and identified by yellow “QUARANTINE” labels (Annexure-1).
-
- If approved material is not used within a specific period, the system will automatically transfer it into the under retest status where suffix ‘Q’ gets affixed to current respective locator code in the system.
-
- For such materials handling refer the SOP of Retesting of raw materials.
-
- Each raw material container/package should have Quarantine labels.
-
- Approved area: After releasing of material in ERP system by QC, relocate the material in the Approved location from quarantine location. And QC shall remove suffix ‘Q’ and shall approve in ERP.
-
- Rejected area: Store all the rejected and expired materials in the rejected area under lock & key condition and identify by “REJECTED” label.
-
- In case the Rejected area is not having sufficient space to store the rejected material, then the material can be stored at other available areas by identifying with the proper rejected label and tied with red rope.
7.0 ANNEXURES:
-
- Raw Material Quarantine/ Under Test Label (Annexure 1)
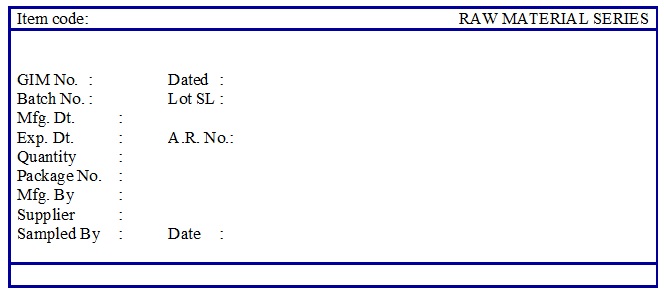
-
- Raw Material Hold Label (Annexure 2)
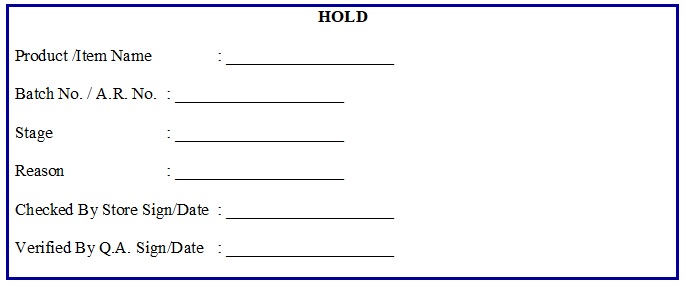
-
-
Receipt cum inspection Report of Raw Material (Annexure 3)
-
| Name of the Item | |||||||||||||||||
| Grade | Item Code | ||||||||||||||||
| Condition of Vehicle | Cleaned | Not cleaned | |||||||||||||||
| Labels on all containers | Present | Not Present | |||||||||||||||
| Condition of container/ box | Cleaned | Not cleaned | Sealed | Broken | Spillage | ||||||||||||
| Transporter Name | |||||||||||||||||
| Docket/LR No. | Date | ||||||||||||||||
| Site | Date of receipt | ||||||||||||||||
| Purchase Order No. | |||||||||||||||||
| Challan No. /Invoice No. & Date | |||||||||||||||||
| Total No. of Packages as per Invoice | |||||||||||||||||
| Total Qty. mentioned in the Challan | Unit | ||||||||||||||||
| Duplicate for transporters copy of Invoice | RECEIVED | NOT RECEIVED | |||||||||||||||
| Party’s approved label defaced | YES | NOT APPLICABLE | |||||||||||||||
| COA Received | YES | NO | |||||||||||||||
| MSDS Received | YES | NO | |||||||||||||||
| Supplier’s Name and site address | |||||||||||||||||
| Manufacturer Name& site address | |||||||||||||||||
| To be stored at | |||||||||||||||||
| Location code | |||||||||||||||||
| Type of Packing | |||||||||||||||||
| GIM No. | Date | ||||||||||||||||
| GIM confirm By | Confirm date | ||||||||||||||||
| Dedusting Done By | Checked By | ||||||||||||||||
| Dedusting Start Time/Date | Dedusting End Time/Date | ||||||||||||||||
| Redressing to be done for Container | |||||||||||||||||
| Balance No.:- |
As per supplier’s claim |
As per physical (wt.) verification |
|||||||
|
Container No. |
Batch/
Lot No. |
Mfg. date | Exp. date | Age of Material | Qty. | Gross wt. | Net wt. | Gross wt. |
Net wt. |
-
-
SHORT-DAMAGED RAW MATERIAL LOGBOOK (Annexure 4)
-
-
-
Raw Material Receipt Authorization Form (Annexure 5)
-
| Name of the Material | |||
| Invoice No. | Item Code | ||
| Mfg. Dt. | Exp. Dt. | ||
| Quantity | No of container | ||
| Date of receipt | |||
| Manufacturer Name | |||
| Supplier Name | |||
| Material details :
……………………… Sign(Warehouse) & Date |
|||
| Justification of acceptance:
……………… Sign(QA) & Date |
|||
-
- GIM FORMAT – RAW MATERIAL (Annexure 6)
-
-
Raw Material Container/Bag Weight Verification Chart (Annexure 7)
-
|
No. of Container Received |
No. of container Weighing performed |
| 10 or less than 10 | All container |
| 11 to 13 | 11 |
| 14 to 18 | 13 |
| 19 to 25 | 14 |
| 26 to 34 |
15 |
| 35 to 45 | 16 |
| 46 to 58 | 17 |
| 59 to 73 | 18 |
| 74 to 90 | 19 |
| 91 to 109 | 20 |
| 110 to 130 | 21 |
| 131 to 153 | 22 |
| 154 to 178 | 23 |
| 179 to 205 | 24 |
| 206 to 234 | 25 |
| 235 to 265 | 26 |
| 266 to 298 | 27 |
| 299 to 333 | 28 |
| 334 to 370 | 29 |
| More than 370 | 30 |



Pingback: Maintenance of Laboratory Instrument - Pharma Beginners
Pingback: GC Column - Receipt, Performance Check and Care - Pharma Beginners