Standard Operating Procedure (SOP) for Operation, standardization, calibration, and maintenance of pH meter including pH meter electrode care.
SOP for Operation and Calibration of pH Meter
1.0 PURPOSE:

-
- The purpose of this SOP (Standard Operating Procedure) is to describe the procedure of operation, standardization, and maintenance of pH meter.
2.0 SCOPE:
-
- This SOP is applicable to the pH meter of the following make and model located in the Quality Control department.
| Make | Model |
| LAB INDIA | PHAN |
| LAB INDIA | PICO + |
3.0 REFERENCES:
-
- Operation Manual supplied by the manufacturer.
-
- SOP: Handling out of calibration results.
-
- SOP: Maintenance of Laboratory Instrument/equipment
-
- SOP: URS and analytical instrument/equipment qualification
-
- SOP: Instrument/equipment/equipment usage log book.
4.0 RESPONSIBILITY:
-
- QC officer shall be responsible for operation, calibration and maintenance of the instrument and its related documentation as per the SOP.
-
- QC Head shall be responsible to provide training to the concerned person before the implementation of the SOP and to ensure the procedure is followed as per the SOP.
Also read: SOP for Operation and Calibration of UV Cabinet
-
- QA shall be responsible to review and check the SOP and to ensure that the procedure is followed as per the SOP.
-
- Plant Head and Quality Head shall be responsible for the approval of the SOP.
5.0 ABBREVIATION:
-
- KCl: Potassium Chloride
-
- NA: Not Applicable
-
- QA: Quality Assurance
-
- QC: Quality Control
-
- RTD: Resistance Temperature Detector
-
- SOP: Standard Operating Procedure
6.0 DEFINITION:
-
- NA
7.0 PROCEDURE FOR OPERATION AND CALIBRATION OF pH METER:
-
-
A) Operation and standardization of pH meter :
-
-
- Precautions during the use of pH meter:
-
- Handle the electrode gently to prevent any type of bulb breakage.
-
- Shake the electrode gently, to ensure that the internal buffer solution covers the whole membrane and no air bubble is entrapped.
Also read: Maintenance of Laboratory Instrument
-
- Wash of any salt film present on the exterior of single rod assemblies using purified water. Do not disturb any pot after standardization.
-
- Ensure that the electrode is stored in 3M KCl or as recommended by the manufacturer if it is not in use.
-
- Do not use the Instrument/equipment more than 24 hours after standardization.
-
- Start-Up of pH meter:
-
- Connect the Instrument/equipment to the power supply and switch the Instrument/ equipment ‘ON’ (When the Instrument/equipment is connected for the first time or after any maintenance, otherwise always keep the Instrument/equipment ‘ON’).
-
-
Preparation of pH Solution and Measurement of pH:
-
-
- Take one capsule content of respective pH buffer capsule (pH-4.0 pH-7.0 pH-9.20) in 10 ml of purified water/CO2 -free water /Milli-Q water and make the volume up to 100 ml with purified water/CO2 -free water /Milli-Q water and dissolve.
Also read: SOP for GC Column Receipt, Performance Check and Storage
-
- Mention the preparation detail in Annexure-3.
-
- Assign In house lot no. to each pH Buffer solution as follows:
-
-
- B.pH value. /XX/YYYY
-
-
-
- Where-
-
-
-
- B-Denote-buffer
-
-
-
- XX-Denote last two digits of the current year
-
-
-
- YYYY-Denote Sr. no. of pH solution
-
-
- Example-B.7.0/20/0001(Buffer of 7.0 pH and serial no. is 0001 and year is 20).
-
-
If required use ready-made buffer solution.
-
-
- Check the standardization status of the Instrument/equipment.
-
- Check the standardization pH range of the electrode. Operate within that range.
-
- If the expected pH of the test falls between the standardization range continue sample pH measurement otherwise standardize the pH meter for the appropriate range.
-
- Prepare samples as per the standard testing procedure or procedure is given for the particular product/material.
Also read: SOP for Disintegration Apparatus (DT)
-
- Adjust the temperature of the solution as given in the individual testing procedure.
-
- Maintain the temperature of the test solution at 25°C ± 2°C, unless otherwise specified in the test procedure.
-
- Remove the electrode and temperature sensor assembly (RTD sensor) and rinse them carefully with distilled water.
-
- Wipe the electrode and sensor with clean dry soft tissue paper without rubbing the bulb of the pH sensor.
-
- Dip the electrode /RTD assembly the sample solution in such a way that the bulb of the electrode sensor is fully immersed in the sample solution.
-
- Press the button “pH” given on the front panel of the Instrument/equipment to record the pH value.
-
-
Allow the reading to stabilize.
-
-
- The display will show the pH value and temperature.
-
- The displayed pH is automatically temperature compensated for span errors in pH sensor when the measurements are taken at temperatures other than 25 °C.
Also read: Operation and Calibration of Analytical Balance
-
- Remove the electrode/ RTD assembly and rinse with purified water.
-
- Shake with a snap action to remove the residual solution.
-
- Store the electrode and temperature sensor in the electrode storage solution only.
-
-
Preparation of 3M KCl:
-
-
- Take 22.35 gm KCl in 100 ml water.
-
- Record all the observations in Annexure-2.
-
-
Conditioning procedure for electrode:
-
-
- Electrode, which has low response due to drying out of the membrane or used under extreme condition may be conditioning by Soaking the electrode in 0.1M HCl 1 hour, followed by immediate rinsing with purified water.
-
- If any other conditioning procedure is recommended by the manufacturer then that procedure shall be followed.
Also read: Analytical Solution Stability
-
- The electrode which fails to respond to the above treatment can be further conditioned by dipping it to 2 % Hydrofluoric acid for 5-10 seconds followed by immediate washing with purified water.
-
-
Standardization procedure ( For PICO + ) :
- Switch ‘ON’ the instrument, the display will show :
-
|
LABINDIA PICO V2.16 pH METER |
-
- Press => ENTER => CAL => ENTER.
-
- The display will show the message ‘ Enter Buffer 1’ Put 4.00 and press ENTER.
-
- Show the message on display ‘ Enter Buffer 2’ Put 7.00 and press ENTER.
-
- The display will show the message ‘ Enter Buffer 3’ Put 9.20 and press ENTER => CE/BACK
-
- Remove the electrode from the buffer solution, wash it with purified water and shake it gently
-
-
Re standardization procedure (for PICO +) :
- Standardize the pH meter with buffer solution 4.0 and after this measure the pH of buffer 4.0 as a sample.
-
Also read: SOP for Rounding off the Analytical Test Results
-
- The observed pH values shall be within ± 0.02 pH unit of the label claim at the measured temperature.
-
- Standardize the pH meter with buffer solution 7.0 and 9.2.
-
- The observed pH values shall be within ± 0.02 pH unit of the label claim at the measured temperature.
-
-
Standardization procedure ( For PHAN ) :
-
-
- Switch ‘ON’ the instrument, the display will show :
|
LABINDIA INSTRUMENTS V1.01 pH ANALYSER |
-
- Standardization for pH meter ( Calibration for Buffer pH 7.0) :
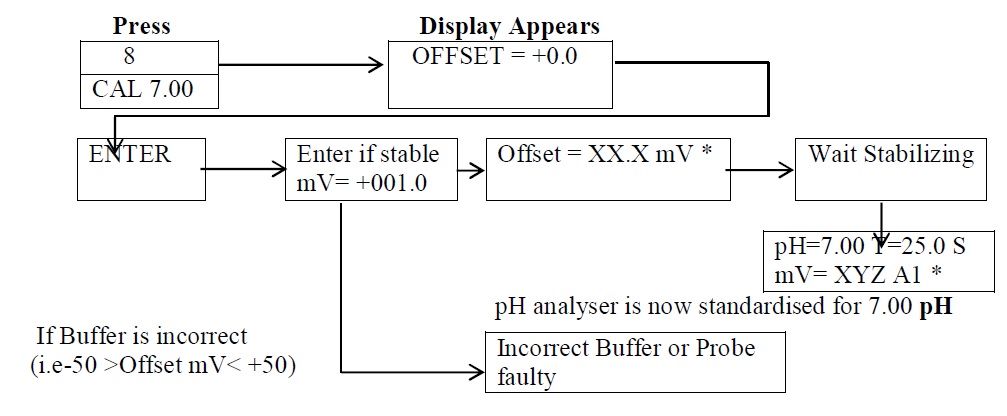
-
- pH meter / analyzer is now standardized for 7.00 pH
-
-
Standardization of pH meter ( Calibration for buffer pH 4.0 ) :
-
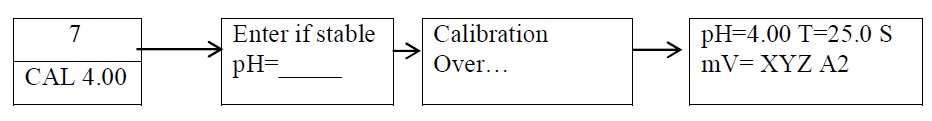
-
-
Standardization of pH meter ( Calibration for buffer pH 9.2 ) :
-
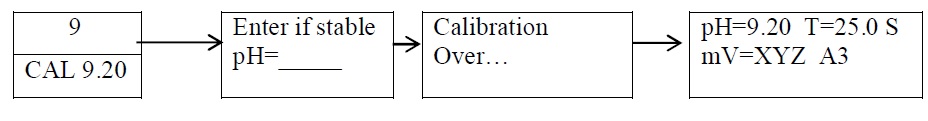
-
- Remove the electrode from the buffer solution, wash it with purified water and shake gently.
-
-
Re-standardization procedure (for PHAN – pH meter) :
- Standardize the pH meter with buffer solution 7.0 and after this measure the pH of buffer 7.0 as a sample.
-
Also read: HPLC Calibration- A Complete Guide
-
- The observed pH values shall be within ± 0.02 pH unit of the label claim at the measured temperature.
-
- Standardize the pH meter with buffer solution 4.0 and 9.2. The observed pH values shall be within ± 0.02 pH unit of the label claim at the measured temperature.
8.0 ANNEXURES OF pH METER SOP:
-
-
Annexure -1: pH Meter calibration template
-
| Instrument/equipment Name: | |||
| Instrument/equipment ID | Make/ Model | ||
| Location | Calibration Frequency | ||
|
Date |
Buffer pH: ____ for Standardization | ||||
| In house Lot No. | Exp. Dt. | pH at 25°C | Temperature displayed (Should be between 25°C ± 2°C) |
pH displayed in Instrument/equipment |
|
| Done By : ( Analyst)
Sign/ Date : |
Checked By : (Reviewer/Designee)
Sign/ Date : |
-
-
Annexure -2 : 3 M KCl Solution preparation template.
-
| Date | Weight taken | Diluted to | Prepared by | Checked by |
Remarks |
-
-
Annexure-3: pH buffer solution preparation log book.
-
|
Sr.No. |
Mfg. Lot No. | Make | pH of buffer solution | Quantity prepared in (ml) | Date of preparation | Use before date | In house lot No. |
Remarks |
-
-
Annexure-4: pH buffer solution stability study template.
-
-
- Objective: To define the procedure for performing the stability study of the pH buffer solution.
-
- Scope: This stability study is applicable for the estimation of the validity of pH buffer solution used at the Quality control Department.
-
-
Procedure:
-
-
- Take one capsule content of respective pH buffer capsule (pH-4.00 pH-7.00 pH-9.20) in 10 ml of purified water/CO2 -free water /Mili Q water and make the volume up to 100 ml with purified water/CO2 -free water /Mili Q water and dissolve.
-
- Note down the preparation record in Annexure-3.
-
- Measure the value and observe the clarity and temperature of the pH buffer solution daily from the date of preparation up to 15 days and report the findings in the format given in Table-1.
-
- In case the manufacturer is changed, the stability study has to be performed.
-
- If a ready-made pH buffer solution is being used, perform the stability study for three months from the date of opening.
-
- The schedule of stability study shall be designed as per the instruction of Head QC.
Table -1
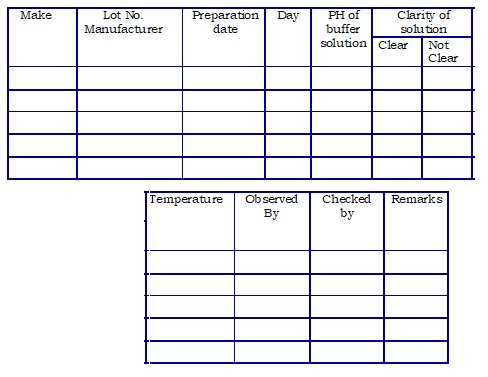
| Name of pH buffer solution | Valid for (in days) : |
| pH |
Conclusion:-
| Prepared By : | Checked: | Approved By: Head QC |


