 Standard Operating Procedure for the handling of Corrective and Preventive Action (CAPA). Corrective and Preventive Action (CAPA) is a concept with current Good Manufacturing Practice (cGMP) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action)
Standard Operating Procedure for the handling of Corrective and Preventive Action (CAPA). Corrective and Preventive Action (CAPA) is a concept with current Good Manufacturing Practice (cGMP) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action)
Handling of Corrective and Preventive Action (CAPA)
1.0 PURPOSE:
-
- This Standard Operating Procedure (SOP) provides the requirements for identification, evaluation, implementation, effectiveness monitoring, closure and documentation of Corrective Actions and Preventive Actions (CAPA).
-
- The CAPA system is designed to address the continuous improvement of Quality Systems.
2.0 SCOPE:
-
- This procedure is applicable for all Corrective and Preventive actions (CAPA) initiated based on information from internal and external GxP systems, processes and records that are recommended at the pharmaceutical manufacturing plant, in any of the following documents, but not limited to:
-
- Annual Product Reviews (APRs)/Product Quality Reviews (PQRs)
-
- Internal and External Audits observation
-
- CAPA identified based on Risk Assessment of the manufacturing process.
-
- Commitments were given to FDA/regulatory bodies as per response to Queries/Audits.
-
- Continual Improvement
-
- CQ CAPA generated as per observation at one plant which shall be applicable to other plants.
-
- Document review like SOPs, MBMR, Specifications & ATP
-
- Equipment & Instrument non-conformity report, Out of Calibration
-
- Event and Investigation
-
- Field Alert Reports
-
- Management Reviews and other sources of quality data/trends.
-
- Market Complaints
-
- Repeat Analysis
-
- Out-of-Specification (OOS) Results
-
- Out-of-Trend (OOT) Results
-
- Product Recalls
-
- Regulatory Inspections/Testing
-
- Regulatory Observations
-
- Process Monitoring
-
- Regulatory recommendation based on a guideline or Pharmacopoeial changes
-
- Risk Management Assessments
-
- Shop floor observations
-
- Stability failure
-
- Validations/Qualification documents
3.0 REFERENCES:
-
- In House
-
- 21 CFR 211.192: Production record review.
-
- USFDA, CDER: Guidance for industry, Quality Systems Approach to Pharmaceutical CGMP Regulations.
-
- ICH harmonized guideline: Pharmaceutical Quality System Q10
-
- EU Guideline for GMP, Volume 4, Chapter 1: Pharmaceutical Quality System
-
- MHRA Orange Book, 2007.
-
- SOP for the investigation in case of final product rejection
4.0 RESPONSIBILITY:
-
-
Corrective and Preventive Action (CAPA )initiator shall be responsible for –
-
-
- Reviewing and initiating the CAPA.
-
- Obtaining reviews from other departments who are stakeholders in developing the CAPA.
-
- Defining the effectiveness criteria, resource requirements to support actions, and the expected completion date.
-
- Assessing the impact on the activities that are to be carried out until the implementation of the CAPA and to build adequate controls during the interim period of CAPA implementation.
-
- Reviewing the status of controls monthly during the interim period.
-
- The corrective/ preventive action, assuring timely completion of implementation activities, tracking progress in completing the CAPA and submitting the CAPA to the QA for review following implementation.
-
-
QA head / designee shall be responsible for-
-
-
- Reviewing and approval of the proposed CAPA, including Supplier and CMO/In-License Third-Party Manufacturers according to Quality Agreements, to assure appropriateness of identified actions and defined timelines.
-
- Assuring CAPA metrics, including trend analysis, are reported to Quality Assurance Management.
-
- The escalation of multi-site/global CAPAs to Quality Review Management.
-
- Selecting the Responsible Person (CAPA Owner), for evaluating site CAPA for multi-site impact.
-
- Approving time extensions for CAPAs that include adequate justification.
-
- Communicating CAPA information, including multi-site impact determinations, metrics, and trends to appropriate individuals at each site, to account management directly responsible for assuring product quality and the prevention of quality issues, to Quality Heads, and to Corporate Quality Compliance.
-
- Compiling CAPA trends and analyzing for further improvements.
-
- Verifying the CAPA implementation is complete and that effectiveness criteria have been met, before closing the CAPA.
-
- Approving completed CAPA and supporting records.
5.0 ABBREVIATIONS – CORRECTIVE AND PREVENTIVE ACTION (CAPA):
-
- APR : Annual Product Review
-
- ATP: Analytical Test Procedure
-
- CCR: Change Control Record
-
- CDER: Centre for Drug Evaluation and Research.
-
- CQ : Corporate QualitY
-
- FDA : Food and Drug Administration
-
- ICH: International Conference on Harmonisation
-
- IT: Information Technology
-
- MBMR: Master Batch Manufacturing Record
-
- MHRA : Medicines & Healthcare Products Regulatory Agency
-
- USFDA: United States Food and Drug Administration
6.0 DEFINITION:
-
-
Corrective and Preventive Action (CAPA):
-
-
- A concept with current Good Manufacturing Practice (cGMP) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action).
-
-
Corrective Action:
-
-
- Action is taken to eliminate the causes of an existing nonconformity, defect or other undesirable situation, in order to prevent a recurrence.
-
-
Preventative Action:
-
-
- Action is taken to eliminate the cause of a potential nonconformity, defect or other undesirable situation, in order to prevent occurrence.
-
-
Contract Manufacturing Organization (CMO)/In-License Third-Party Manufacturer:
-
-
- An organization that supports any part of, or completes the process of manufacturing, labeling, packing, testing, and distribution of product on behalf of another organization. Contract manufacturing involves the production of goods by an organization, under the label or brand of another organization.
-
-
Correction:
-
-
- Action taken to eliminate non-conformity.
-
-
Effectiveness Criteria:
-
-
- Measurable standards that, if met, demonstrate a corrective action has prevented recurrence of a finding/issue and/or a preventive action has prevented a potential finding/issue before it occurred.
-
-
Effectiveness Monitoring:
-
-
- A post-execution assessment of CAPAs to verify that implemented actions have the desired outcome, as defined by the success criteria.
-
-
Quality Council /Quality Review Management:
-
-
- A forum to support the premise that the Quality Council is a mechanism to exercise management responsibility, as well as to ensure timely decisions and cross-functional support.
-
- It is a forum for leadership engagement, awareness and decision making around quality systems and process/ product performance.
-
- It is not a substitute for line management accountability or the only forum for addressing improvements to quality systems and cGMP problem-solving.
-
-
Subject Matter Expert:
-
-
- An individual, who is educated, trained, experienced, and recognized in a particular field or subject matter.
-
-
cGxP:
-
-
- cGxP is a general term that stands for current Good “x” Practice (x = Clinical, Engineering, Laboratory, Manufacturing, Documentation, Pharmaceutical, etc.).
-
- The titles of these Good “x” Practice guidelines usually begin with “Good” and end in “Practice”. cGxP represents the abbreviations of these titles where “x” a common symbol for a variable, represents the specific descriptor.
7.0 PROCEDURE FOR HANDLING OF CORRECTIVE AND PREVENTIVE ACTION (CAPA):
-
-
General Instruction:
-
-
- A Corrective and Preventive Action (CAPA) may initiate as an outcome of, but not limited to-
-
-
- Process Monitoring,
-
-
-
- Regulatory Inspections/Testing, Deviations,
-
-
-
- Internal/External Audit Observations,
-
-
-
- OOS Results,
-
-
-
- OOT Results,
-
-
-
- Product Quality Complaints,
-
-
-
- Product Recalls,
-
-
-
- New Amendments to regulatory Guidelines,
-
-
-
- Returned Products,
-
-
-
- Investigations,
-
-
-
- Product Quality Reviews,
-
-
-
- Risk Assessments,
-
-
-
- Management Reviews,
-
-
-
- Any Warning Letters or other Regulatory Observations,
-
-
-
- Negative GMP Trends and other sources of Quality data.
-
-
-
- CAPAs shall be initiated by QA or Other Departments.
-
-
-
Corrective and Preventive Action (CAPA) Initiation:
-
-
- On receipt of approved documents (e.g. documents as mentioned under scope), QA supervisor shall list down the recommendation or suggestive Corrective Action or Preventive Action and update the Corrective and Preventive Action (CAPA) register (Refer Annexure-2).
-
- Corrective and Preventive Action (CAPA) can initiate by any department.
-
- The Initiator shall open CAPA(s) record(s) for each set of corrective action(s) and preventive action(s), respectively, and each CAPA initiated shall be identifiable with a unique identification number.
-
- CAPA numbering system : CAPA/YY/ZZZ
-
- Where,
-
- First four characters – the prefix CAPA followed by a slash(/)
-
- Next two Characters, i.e. YY, last digits of the current year (YY) followed by a slash (/).
-
- e.g. Like 20 for the year 2020, 21 for the year 2021 and so on…….
-
- Last three digits, i.e. ZZZ, the serial number in chronological order beginning from 001, 002, 003 and so on …….. this serial number shall start from 001 from 1st January of each year.
-
- e.g. First CAPA of the QC department for the year 2020 shall be numbered as CAPA/20/001.
-
-
Note: If Corrective action and/or preventive action to be taken for different documents are same then one common CAPA No. can be issued. This numbering system is for guidance purposes only and CAPA numbering can be changed company to company.
-
-
- The following information shall be provided for the CAPA as applicable: Initiator’s Name, Department/Block/Area Name, short description of Quality issue, CAPA priority, Source of CAPA, type of activity (corrective or preventive action), description of the CAPA, Justification, CAPA due date, root cause category and a summary of root cause.
-
- Attache all relevant data and files needed to support the source of CAPA.
-
- QA designee shall make entries into CAPA register as mentioned below:
-
-
- Initiator: Name of Initiator
-
-
-
- Department: Name of initiator department
-
-
-
- Issued on: The date on which the Corrective and preventive action assigned.
-
-
-
- Issued By: Name of QA supervisor by whom CAPA form is assigned.
-
-
-
- Document Type: The reference document type in the context of which above corrective action assigned
-
-
-
- Reference Document No.: The reference document number in the context of which above corrective action assigned
-
-
-
- Product /Material /System/Reference document Name: Name of Product / Material / Document title in the context of which above corrective action assigned
-
-
-
- Reference Batch No./ Effective Date: The Reference Batch No / Effective date in the context of which above corrective action assigned
-
-
-
- Recommendation: The recommendation details specified.
-
-
-
- Rationale: In this section rational for CAPA generation shall be mentioned. e.g.: Based on trials taken for effectiveness, literature/References, etc.
-
-
-
- Responsible department: The department responsible for the Implementation of Corrective and preventive action.
-
-
-
- Responsible Person: The name of the responsible person for the completion of the recommendations shall be written in consultation with the concern department head by QA
-
-
-
- Target completion Date: The target completion date is the date by which the recommended activities to be completed in consultation with the concern department head.
-
-
-
Note: In case of more than one recommendation having different target completion dates, the target date of particular Corrective and Preventive Action (CAPA) shall be assigned whichever is later.
-
-
- Extended target completion date: Mention the new tentative extended target completion date.
-
- Status: On the basis of the fulfillment of the ‘status’ whether ‘open’ or ‘close’ determine.
-
- Closed on: Closing Date of CAPA.
-
- Remarks: Write the remark if any.
-
- QA designee shall make entries into the CAPA register (Annexure 2).
-
- CAPA Request form (Refer Annexure 3) shall be filled up by QA- designee and issued to the concerned department.
-
- The Corrective and Preventive Action (CAPA) initiator may further send out for department review if required.
-
- The Initiator shall submit the completed CAPA record to the QA for review and evaluation.
-
- QA shall return the CAPA record to the Initiator in case any additional information is required.
-
- Upon satisfactory review of the CAPA details, the QA shall submit the CAPA record to the Quality Review Board (QRB) Chair.
-
- The Quality Review Management shall review the following (but not limited to):
-
-
- Adequacy of Corrective and Preventive Action (CAPA) scope,
-
-
-
- Timelines,
-
-
-
- Root cause(s),
-
-
-
- Proposed corrective/preventive actions and
-
-
-
- Potential for multi-site impact.
-
-
- The QRB chair / or designee shall discuss with relevant stakeholders and department heads prior to assigning the Responsible Person (Corrective and Preventive Action (CAPA) Owner) for implementation of CAPA.
-
- The QRB chair / or designee shall assign CAPA tasks to RPs where the multi-site impact has been determined.
-
-
Implementation of Corrective/Preventive Action:
-
-
- The CAPA Owner(s) shall define the effectiveness criteria, resource requirements, tentative date/time for completion, and effectiveness monitoring period.
-
- The CAPA owner(s) shall perform an impact assessment to evaluate the impact on product quality, supply and product safety, as well as the impact on other systems and processes by the activities that are to be carried out until implementation of the CAPA is complete. Where applicable,
-
- The Owner shall also establish adequate controls during the interim period prior to CAPA implementation.
-
- Review the status of controls on monthly during the interim period.
-
- The CAPA owner shall set the CAPA implementation priority to be commensurate with risk determined in the impact assessment.
-
- The CAPA owner shall implement corrective/preventive action.
-
- The functional supervisor/department head of the CAPA Owner shall ensure that adequate resources are provided for timely CAPA implementation.
-
-
The Corrective and Preventive Action (CAPA) Owner shall initiate CCR to achieve effective implementation of CAPA.
-
-
- Upon completion of the recommended activity concerned department shall write down the details of action taken along with supporting data, if any after verification from the department head or designee.
-
- The person performing the assigned Corrective and preventive action shall sign in the performed by column and the respective person of the concerned department verifying the above action shall sign in the verified by column
-
- In the traceability matrix, complete traceability of Corrective and Preventive Action (CAPA) to the implemented document shall be provided.
-
- After completion of the above activity the duly filled and signed CAPA Request Form and submit to the QA department for evaluation along with supporting data/documents.
-
- The Corrective and Preventive Action (CAPA) Owner shall review and document activity implementation and report evidence that the corrective/preventive actions were effective along with the date of CAPA implementation.
-
-
Status updates concerning effectiveness monitoring are provided to the QA on a quarterly basis.
-
-
- QA shall review the CAPA register on a monthly basis to identify the CAPA request for closing or the target date for due for closuring.
-
- During implementation, if it is necessary to discontinue the Corrective and Preventive Action (CAPA) for any scientifically justifiable reason (e.g. discontinuation of the product, closure of facility/area/unit),
-
- The CAPA Owner shall send a discontinuation request with justification to the RP – QA.
-
- The CAPA shall be discontinued and closed if supporting justification is determined to be acceptable and closure is approved by QA.
-
- However, if the supporting justification provided by the CAPA owner is not satisfactory, it shall be returned to the CAPA owner for implementation.
-
-
Corrective and Preventive Action (CAPA) Changes:
-
-
- Changes shall be addressed by closing the CAPA with appropriate justification, including QA approval and opening a new CAPA with a cross reference to the closed CAPA.
-
- Closing an existing CAPA and opening a new CAPA shall be required for any of the following reasons as applicable:
-
-
- Change in scope
-
-
-
- Change in acceptance criteria
-
-
-
- The significant change to the approved action plan
-
-
-
- Change in the effectiveness monitoring plan
-
-
-
- Implementation of alternate solutions shall require a re-evaluation of the potential risk assessment.
-
-
-
- Changes shall include review and revision of interim control measures to minimize risk, where applicable.
-
-
-
- Major changes to CAPAs resulting from a Deviation record shall be communicated to and approved by QA.
-
-
-
- Changes to CAPAs resulting from Inspection Commitment require the necessary escalation to Site, Regional and/or Global levels as it is part of the commitment to Regulatory Agencies.
-
-
-
Track CAPAs for closure against assigned due dates.
-
-
- QA shall review the CAPA register on monthly basis to identify the Corrective and Preventive Action (CAPA) request which needs to be closed or the target date for closure is due, prior to a Corrective and Preventive Action (CAPA) being overdue and communicate to CAPA owner.
-
- The CAPA Owner may also seek an extension of the closure due date by providing supporting justification to QRB Chair for concurrence.
-
- The justification for extension shall be prepared by the concerned department and sent for checking to the concern department head and QA department/site quality head.
-
- The request for extension shall be supported with adequate justification/rationale and include the results of an impact assessment related to the delay in Corrective and Preventive Action (CAPA) implementation.
-
- The Corrective and Preventive Action (CAPA) due dates are assigned mutually by the QRM chair in consultation with relevant stakeholders.
-
-
The Corrective and Preventive Action (CAPA) due to date extensions may vary for each CAPA based on its nature.
-
-
- In the case of Corrective and Preventive Action (CAPA)where the collection of extensive data or consultation with some external agency is required, the target completion date may be extended with a justified reason after approval by Quality Head.
-
- Quality Head shall approve the justification of the target completion date extension.
-
- The Site Quality Head or designee shall write the next target date in the next column.
-
- Management Notification (Corporate Management: Site Quality Head shall raise if the third extension in the target completion date is required.
-
- Post-implementation of Corrective and Preventive Action (CAPA), the QA shall verify the effectiveness of Corrective and Preventive Action (CAPA) based on the effectiveness criteria determined earlier.
-
-
CAPA Effectiveness monitoring:
-
-
- CAPA shall verify or validate to assure that such actions are effective and do not adversely affect product quality or process.
-
- Effectiveness monitoring shall use a method or approach that is based upon the established success criteria.
-
- Perform the effectiveness of checks/verifications in the following ways (but not limited to):
-
- Monitoring of quality data of batches (No. / Period).
-
- Implementation after training in case of SOP revision.
-
- Monitoring of event after implementation of Corrective and Preventive Action (CAPA) action(s) for occurrence/re-occurrence for a suitable period.
-
- Effectiveness check is required for Corrective and Preventive Action (CAPA) initiated due to Regulatory Inspection Observation, Critical and Major Quality Events/Deviations, Market Complaints, Recalls and OOS for marketed products.
-
- Proof of effectiveness shall be planned, performed, and documented for all CAPAs. Proof may be demonstrated by performing a verification/validation of the improved process, by monitoring the process over an extended period of time according to the approved acceptance criteria for effectiveness or by other appropriate means.
-
- Re-evaluate causes and solutions, a new action plan shall be developed and approved by QA when effectiveness monitoring results do not meet predefined success criteria.
-
- QA shall approve and document any proposed rationale for not performing proof of effectiveness.
-
- The mechanism shall be established to communicate status update to QA Management and the Corrective and Preventive Action (CAPA) Owner when Corrective and Preventive Action (CAPA) effectiveness monitoring becomes overdue.
-
- The frequency of status updates shall be determined by the Corrective and Preventive Action (CAPA) Owner and shall not exceed quarterly updates.
-
- Status updates during the Effectiveness Monitoring phase shall be made quarterly, at a minimum, if the target effectiveness monitoring completion date is greater than ninety (90) days.
-
-
Corrective and Preventive Action (CAPA) closure:
-
-
- QA shall verify all items adequately addressed during review and approval of Corrective and Preventive Action (CAPA) closure, including, but not limited to:
-
-
- Completion of all required steps.
-
-
-
- Corrective and Preventive Action (CAPA) quality and compliance objectives have been met;
-
-
-
- Documentation of all decisions and actions, Approval of QA.
-
-
-
- Verification that any revisions of approved follow-up activities are traceable to the original CAPA.
-
-
-
- Completion of proof of effectiveness.
-
-
-
- Assurance that potential multi-site impact assessment findings have been documented and communicated to CQC to address issues, as needed.
-
-
-
- CQC shall close CAPAs where the multi-site impact was determined.
-
-
-
- Closure of Change Control completed.
-
-
- If the Corrective and Preventive Action (CAPA) implementation is found to be satisfactory by QA, based on the established effectiveness criteria and potential multi-site impact assessment, decisions have been documented and communicated to CQC, the CAPA shall be closed.
-
- If the Corrective and Preventive Action (CAPA) implementation is found to be ineffective, QA shall return the CAPA to the CAPA Owner (Responsible Person) for further actions with a review summary.
-
-
Multi-site/Global Corrective and Preventive Action (CAPA):
-
-
- QA/QRB Chair shall notify CQC when a Corrective and Preventive Action (CAPA) review and approval indicates a potential for multi-site impact (includes implementation of new requirements or major changes to existing requirements).
-
- Perform the evaluations for potential multi-site impact and decisions.
-
-
The QRM chair or designee shall:
-
-
- Monitor the effective and timely implementation of the Corrective and Preventive Action (CAPA).
-
- Compile Corrective and Preventive Action (CAPA) trends and analyze for further improvements.
-
- In the event of a potential multi-site impact, the QRB shall communicate the Corrective and Preventive Action (CAPA) to (CQC) who shall evaluate the impact of the Corrective and Preventive Action (CAPA) and select Regional Quality Heads and respective sites for evaluation.
-
-
Corporate Quality Compliance shall:
-
-
- Notify the Regional Quality Heads, as applicable, of the multi-site impact determination.
-
- Monitor the overall completion of multi-site Corrective and Preventive Action (CAPA)s and track completions to closure.
-
- Review and evaluate potential quality or multi-site impact.
-
- Assure actions taken by the sites in response to the issue are coordinated to ensure the issue is systematically addressed.
-
- Monitor the status of multi-site CAPA on a monthly basis.
-
- Assure all Corrective and Preventive Action (CAPA)-related decisions are approved by Quality and documented.
-
-
Requirements:
-
-
- Implement a tracking system approved by QA to support the CAPA control system.
-
- Corrective and Preventive Action (CAPA) date closures against assigned targets and effectiveness metrics shall be reported to the appropriate site or senior management.
-
- CAPA shall be planned, implemented and documented:
-
- The implementation shall be in accordance with approved change control procedures where the Corrective and Preventive Action (CAPA) involves a change.
-
- Predefined Corrective and Preventive Action (CAPA) acceptance criteria shall be established in order to develop the appropriate action plan and effectiveness monitoring plan.
-
- Each issue shall be assessed for significance and risk to product quality, supply and overall safety of the affected product(s).
-
- The priority and timelines for the Corrective and Preventive Action (CAPA) implementation shall be commensurate with the level of risk.
-
- Action plans shall consider the potential impact to other systems and processes (e.g. validated systems or equipment, regulatory commitments, regulatory submissions, as well as those of third parties).
-
- CAPA implementation shall take into account potential risk as the CAPA action plan is developed and shall include interim controls to assure product quality prior to CAPA implementation.
-
- CAPA detail falling within the scope of Annual Product Reviews (APRs) and Product Quality Reviews (PQRs) shall be submitted to Quality Review Board and communicated to Regulatory Affairs, as required.
8.0 ANNEXURES:
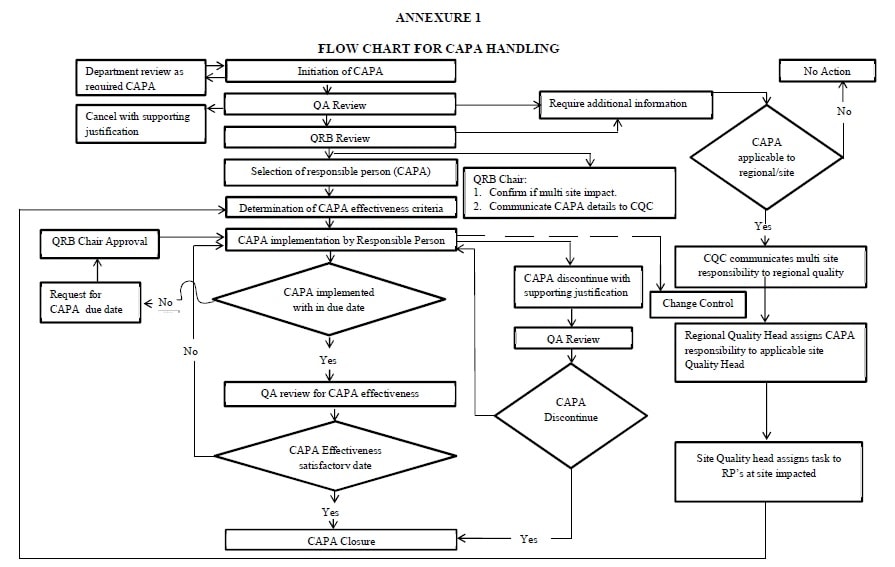
Annexure 1: Flow chart of CAPA Handling.
Annexure 2: CAPA Register.
Prepare the CAPA log/register with the following table contents…
-
- CAPA No.
-
- Issued On
-
- Issued By
-
- Source of CAPA
-
- Reference Document No.
-
- Product / Material / Reference Document No.
-
- Reference Batch No. / Effective Date
-
- Responsible Department
-
- Responsible Person
-
- Corrective actions
-
-
- Proposed Corrective actions
-
-
-
- Target completion Date
-
-
-
- Extended target completion date
-
-
- Preventive actions
-
-
- Proposed Preventive actions
-
-
-
- Target completion Date
-
-
-
- Extended target completion date
-
-
- Effectiveness review required (Yes / No)
-
- Status (Open / Closed)
-
- Closed On
-
- Remarks
Annexure 3: CAPA Request Form.
| CAPA No. | Issued on | ||
| Department | Issued by
( Sign & Date) |
| Source of CAPA | |||
| Product / Material / Document / System name | Reference document no. | ||
| Ref. Batch No. / Effective date | |||
| Responsible Person | |||
| Proposed Corrective Actions : | Target Completion Date | Extended Target Completion Date | ||||
| Proposed Preventive Actions : | Target Completion Date | Extended Target Completion Date | ||||
| Notify by | Comment | Sign & Date | ||||
| Plant Head | ||||||
| Approval | Comment | Sign & Date |
| User Dept. Head | ||
| QA Head/Designee | ||
| Site Quality Head |
Implementation status |
|||||
| Corrective Actions Taken | Implementation on Date | Reference document No. | |||
| Preventive Actions Taken | Implementation on Date | Reference document No. | |||
Traceability Matrix |
|||||
| Product Name / Material Name | |||||
| Document Name (Type) | |||||
| Document No. | |||||
| Section / Page No. / Step No. | |||||
| Ref. Batch No. / Effective date | |||||
| Any other (Specify) | |||||
| Supporting Data Attached | Yes / / No | ||||
| Responsible Person
(Sign & Date) |
|
Verified By
(Sign & Date) |
|||
Evaluation and closure by QA Department |
||||
| Remarks of QA (Evaluator) :
Evaluated By (Sign & Date) |
||||
| Effectiveness Review required : Yes / No
Remark : QA Head (Sign & Date) |
||||
| Remark for closure :
Closed By (Sign & Date) |
||||
| Justification of Target Completion Date Extension | ||||
| CAPA No. | ||||
| Document No. | ||||
| Document Details | ||||
| Initial Target Date | ||||
| Extension No. | 1 | 2 | 3 | |
| Extended Target Date | ||||
| Current status and
Pending activities of proposed CAPA |
||||
| Justification for Extension | ||||
| Prepared by
(Sign & Date) |
Concern Department | |||
| Checked By
(Sign & Date) |
Concern Dept. Head | |||
| QA Department | ||||
| Approved By
(Sign & Date) |
Site Quality Head | |||
Annexure 4: CAPA Discontinuation Request.
| CAPA No. | Date of issuance | ||||
| Type of Change | Department | ||||
| Proposed Corrective Action | |||||
| Proposed Preventive Action | |||||
| Justification for discontinuation | |||||
| Supporting Document (If any) | |||||
| Prepared by
Sign & Date |
Reviewed by
Dept. Head Sign & Date |
Approved by
QA Head/ Quality Head Sign & Date |
|||
Format for CAPA Effectiveness Verification
| Section: CAPA Effectiveness Verification | |
| CAPA Effectiveness Verification (Required/ Not Required) _______________ | |
| Reason If CAPA Effectiveness Verification Not Required: | |
| QA Head (Sign/Date) | |
| CAPA No.: | |
| Section XII: 1st CAPA Effectiveness Verification | |
|
|
| Initiator Dept. Head (Sign/Date) | |
|
|
| Reference No. for investigation, if CAPA is Not Effective | |
| New CAPA No. Against not effective CAPA. | |
| QA In-charge
(Sign/Date) |
|
|
|
| QA Head (Sign/Date) | |
| NOTE: This page shall be issued separately for Effectiveness verification of CAPA initiated before revision of this format. | |
| CAPA No.: | |
| Section XII: 2nd CAPA Effectiveness Verification | |
|
|
| Initiator Dept. Head (Sign/Date) | |
|
|
| Reference No. for investigation, if CAPA is Not Effective | |
| New CAPA No. Against not effective CAPA. | |
| QA In-charge (Sign/Date) | |
|
|
| QA Head (Sign/Date) | |
| NOTE: This page shall be issued separately for Effectiveness verification of CAPA initiated before revision of this format. | |
*************************************END****************************************



Pingback: SOP for Corrective Action in Pharmaceuticals Industry - Pharma Egg
Pingback: Good Clinical Practices (GCP) Guideline - Pharma Beginners
Pingback: Media Fill Test - Aseptic Process Simulation in Micro - Pharma Beginners
Pingback: Out of Specification Result in Microbiology - Guideline - Pharma Beginners
Pingback: SOP for Line Clearance (LC) of Area and Equipments - Pharma Beginners
Pingback: Incident Handling and Investigation Procedure - EHS - Pharma Beginners
Pingback: Analytical Method Transfer (USP 1224) Guideline - Pharma Beginners
Pingback: Cross Contamination, Mix-Ups & Microbial Contamination - SOP in Pharma