SOP for handling and reporting of Laboratory Incident (Lab Event ) occurs in the Quality Control like be-Bracketing standard failure, Improper peak shape, Extraneous peak, baseline disturbance, peak elution pattern change, Data missing, wrong standard/sample weight taken, wrong standard/sample preparation, wrong wavelength selection, wrong analytical column selection, etc.
SOP for Handling and Reporting of Laboratory Incident/Lab Event
1.0 Purpose:
-
- To define the procedure for handling of Laboratory Incident(s) in quality control departments.
2.0 Scope:
-
- This SOP is applicable to any investigation which does not qualify the requirement of OOS and OOT. This can be like…
-
-
- Bracketing standard failure,

- Bracketing standard failure,
-
-
-
- Improper peak shape,
-
-
-
- Extraneous peak,
-
-
-
- Baseline Disturbance,
-
-
-
- Peak elution pattern change,
-
-
-
- Data missing,
-
-
-
- Wrong standard/sample weight taken,
-
-
-
- Preparation of wrong standard/sample,
-
-
-
- Wrong wavelength selection,
-
-
-
- Selection of wrong Analytical column (but not limited to) that occurs in the Quality control lab.
-
3.0 Reference and Annexures :
-
-
References :
-
-
- SOP for Operation and Calibration of the HPLC system
-
- SOP for GC Column Management.
4.0 Responsibilities – Laboratory Incident:
-
-
Analyst :
-
-
- To immediately inform the Head QC/ Section Head or designee about Laboratory Incident and shall not discard any solution preparation, glassware used and instrument settings until the evaluation of the Laboratory Incident.
-
- To participate in the investigation, participate in finding of root cause and to carry out the experimental analysis where applicable.
-
-
Section Head or designee:
-
-
- Carry out the investigation with the concerned analyst as per the SOP.
-
- Plan hypothesis study as and when required to identify the root cause.
-
- Review historical data of laboratory investigations during the initial assessment to determine if similar laboratory Laboratory Incident has been occurred previously, what were the corrective action and preventive actions and the effectiveness of CA & PA.
-
- To initiate the actions recommended in Laboratory Incident Form.
-
- Based on assignable cause determination of CA & PA and perform impact & Risk assessment and training.
-
- Periodic review of Lab Incident Trends and evaluation of the effectiveness of CA and PA derived based on Laboratory Incident Trends.
-
- Head QC or Designee:
-
- Provide guidance for investigation.
-
- Review and conclude the Laboratory Incident Form.
-
- Initiate the actions recommended in the Laboratory Incident Form.
-
- Review and approve the hypothesis study protocol and report.
-
- Based on assignable cause determination of CA & PA and perform impact & Risk assessment and training.
-
- Periodic review of Lab Incident Trends and evaluation of the effectiveness of CA and PA derived based on Lab Incident Trends.
-
-
QA of QC (GLP Personel) :
-
-
- To register the Lab Incident in “ Laboratory Incident form issuance register” as per the SOP.
-
- Issuance of the Laboratory Incident Form.
-
- To provide guidance for the Lab Incident investigation.
-
- Ensure the compliance of CA & PA mentioned in the Laboratory Incident Form.
-
- Perform periodic trending and review of Lab Incident (s) and evaluation of the effectiveness of CA & PA derived based on Lab Incident Trends.
-
-
Head QA or designee :
-
-
- To ensure implementation of CA & PA recommended in the Lab Incident Form.
-
- To ensure the implementation of the system as per the SOP.
-
-
Head Quality :
-
-
- Review and approve the Lab Incident Form.
-
- Review and approve the SOP.
-
- Ensure the implementation of the system as per the SOP.
-
-
Regulatory affairs and Plant Head:
-
-
- To review and approve the SOP.
5.0 Abbreviations& Definition of Terms:
-
-
Abbreviations:
-
-
- ATP: Analytical Test Procedure.
-
- CA: Corrective Action.
-
- FMEA: Failure Mode Effect Analysis.
-
- GC: Gas Chromatography.
-
- HPLC: High-Performance Liquid Chromatograph.
-
- OOT: Out of Trend.
-
- OOS: Out of Specification.
-
- PA: Preventive Action.
-
- QC: Quality Control.
-
- QA: Quality Assurance.
-
- SME: Subject Matter Expert.
-
- Definition of Terms :
-
-
Assignable Cause:
-
-
- A cause that can be attributed as the root cause for the Lab Incident. The assignable cause is a conclusion derived from direct or indirect evidence found during the investigation process, from the interpretation of analytical data or a combination of both.
-
-
Hypothesis Study:
-
-
- The structured documented sequence of experiments that are designed to identify the root cause for the Lab Incident.
-
- This shall be based on discussions, thought process and scientific rationales, leading towards identifying the potential root cause (s).
-
- Each experiment shall have the pre-defined expectations of the actual outcome to be observed through that particular experiment.
-
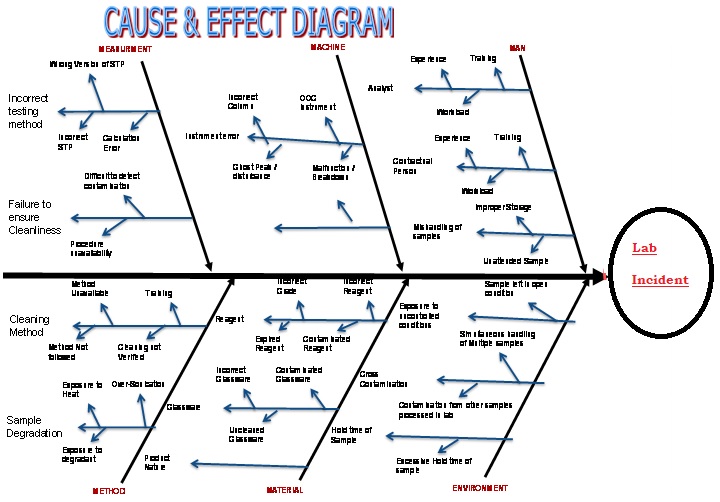
- Fish Bone Diagram. ( Annexure –5) can be used as an Investigational tool for Hypothesis ( Annexure – 6)
-
-
Investigation:
-
-
- Review and experiments conducted by investigators [Team of ‘Subject Matter Experts (SME’s)] and the analyst to find the root cause attributing to Lab Incident.
-
- This could be a laboratory and/or cross-functional investigation.
-
- This should be thorough, timely, unbiased well documented and scientifically justified.
-
-
Laboratory Incident:
-
-
- A Lab error that does not qualify for out of specification or out of trend results shall be considered as Lab Incident.
-
- Laboratory errors that are generated due to deviation to the stated standard operating procedure, analytical test procedure shall not be considered as a Lab Incident/lab event and those shall be registered and investigated as per SOP for “Event reporting and investigation”.
-
-
Out of Specification:
-
-
- Test results that fall outside the established approved specification limit or acceptance criteria.
-
- CA (Corrective Action): Action to be taken to correct the cause and avoid the re-occurrence.
-
- PA (Preventive Action): Action to be taken to avoid the occurrence.
-
-
Repeat analysis:
-
-
- Repeat the analysis by repeating one or more steps of the sample preparation from the original preparation or fresh sample preparation.
-
- A repeat analysis may include the preparation of fresh standards and/ or other test reagents as appropriate.
6.0 Procedure for Handling and Reporting of Laboratory Incident:
-
- In case of any Lab Incident observed, during analysis /raw data review, the analyst shall immediately inform to section head or designee and shall not destroy any solution/ sample/ instrument setup until the evaluation of the Lab Incident/lab event completed by section head or designee.
-
- Note: Analyst should be aware of a potential problem (s) that could occur during the testing and should watch for problems that could create inaccurate results.
-
- The analyst should not knowingly continue any analysis which he or she expects to invalidate at a later stage due to any assignable cause.
-
- Section head or designee shall inform to QA of QC. QA of QC shall take the following actions as under,
-
- Issue “ Laboratory Incident Form” (refer Annexure-2) to the analyst and make relevant entries in the “Laboratory Incident Form Issuance Register” (refer Annexure-1) and “Laboratory Incident Form” (refer Annexure-2).
-
-
QA of QC shall issue the “ Laboratory Incident form” with proper numbering which shall be as follows:-
-
-
-
- LIF/YY/XXX,
-
-
-
- Where, LEF = Laboratory Incident Form
-
-
-
- YY = Current year (e.g for the year 2020, YY will be 20 )
-
-
-
- XXX = Serial No. starting from 001
-
-
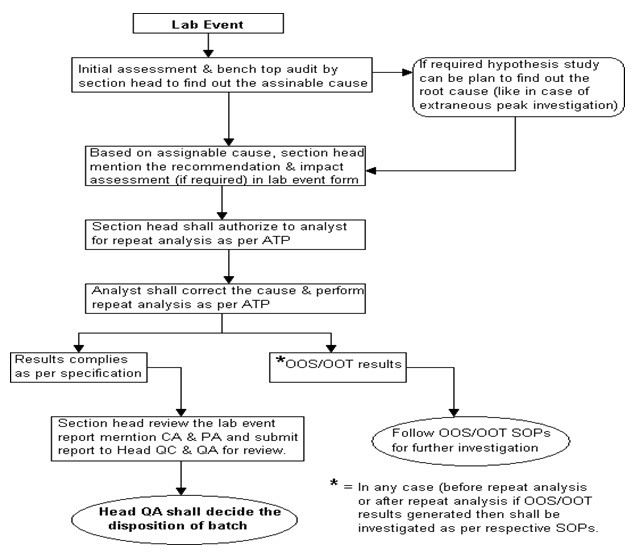
- Section head or designee shall perform an initial assessment, discuss with the analyst and perform benchtop /on the spot audit and documentation (as a part of the investigation) to find out the root cause/assignable cause.
-
- Based on the root cause identified, Section Head or designee shall mention “recommendation” and “impact assessment” in Laboratory Incident Form.
-
- Note: Lab Incident, in general, will have a known assignable cause and if any hypothesis testing is required to identify or establish the root cause, then the hypothesis study shall be planned with approved hypothesis protocol (protocol shall have predefined objective).
-
- After authorization of section head or designee & verification of QA of QC, the analyst shall correct the cause and perform further analysis as per STP with the same analysts.
-
-
Note: In the case of the same analyst is not available then analysis can be allotted to another analyst After authorization of Head QC/ designee.
-
-
- The analyst shall report the result and submit to the section head or designee.
-
- Based on the outcome of the result, section head or designee shall decide further action as under,
-
- If the result complies with the approved effective specification limit, report the result, mention conclusion, CA & PA in incident form and submit the report to Head QC/designee & Head QA/designee for disposition of material.
-
- In any case, if results generated are found “Out of specification” or “Out of trend” then investigation shall be registered and investigated as per the respective SOP i.e. “Handling of out of specification” or “Handling of Out of trend results”.
-
-
Trending and evaluation of Laboratory Incident:
-
-
- Trending and evaluation of Incident/Lab Event shall be performed by QA of QC on a quarterly basis to identify the repetitive type of Incident/lab event and necessary CA & PA shall be derived.
-
- Head QC or designee shall assess the effectiveness of CA & PA derived based on Incident/lab event trends.
-
-
General Procedure for Handling of Laboratory Incident:
-
-
- Incident investigation shall be completed within 15 days from the date of the registered.
-
- In case the Incident investigation is not closed within 15 days then authorization with justification for the extension of investigation completion shall be taken from Head QC or designee and shall be recorded in the “Laboratory Incident form”.
-
- Any Lab Incident shall not be open beyond 30 days.
-
- In case re-sampling is required as a part of the investigation/analysis, then re-sampling shall be performed after proper justification and authorization of Head QA on Annexure-2.
7.0 Annexures :
Laboratory Incident Form Issuance Register. (Annexure-1)
Laboratory Incident Form (Annexure-2)
| Laboratory Incident No. : | Issued by /Date: | The target for completion date: | |
| Issued to : | |||
| Product / Material: | |||
| Batch No./A.R.No : | |||
| Stage : | |||
| Test : | |||
| Initial Results: | Limit : | ||
| Reference of Raw data : | |||
| Laboratory Incident/Event details : | |||
Investigation Checklist (but not limited to),
| Sr. No. | Parameters | Observation | Sign / Date | |
|
1 |
Checked condition of the sample. (Check for Physical examination, storage condition, Storage container, labeling etc.) |
|||
|
2 |
Balance checked for its calibration.
Code No. of Balance : |
|||
|
3 |
Instrument Calibration checked.
Name of Instrument : Code No.: |
|||
|
4 |
Reagents used for analysis checked.
(Check for raw data, physical appearance, and validity of reagent used.) |
|||
|
5 |
Checked for dilution, calculation, weighing Titer values and readings. |
|
||
|
6 |
Working standard checked (Check for physical condition, validity.)
Code No. of WS : |
|||
|
7 |
Chromatograms / printouts / TLC plates checked. |
|
||
|
8 |
System suitability checks (Chromatography Analysis) |
|
||
|
9 |
Check the bracketing standard for RSD. |
|
||
|
10 |
Checked for Method of analysis followed.
Method Reference No.: |
|||
|
11 |
Any Other(s) : |
|
||
| Initial assessment: | |
| Recommendation : | |
| Impact assessment: | |
| Authorized by /Date
Section head or designee : |
Verified by /Date:
Head QA/ designee : |
| Analysis allotted to/ date: | |
MATERIAL /PRODUCT RESAMPLE AUTHORIZATION FORM
| Justification for Re-sampling: | ||||
| B No. / AR NO.: | For the investigation stage | QTY.: | Authorized By/Date: (Head QA) | Sampled By/Date: |
|
|
||||
| Repeat analysis Results: | Raw data reference: | ||
| Justification/reason for the extension of the investigation | Sign/Date
(Head QC) |
||
| Final conclusion: | |||
| Corrective action:
(CAPA No.:________ ) |
|||
| Preventive action:
(CAPA No.:________ ) |
|||
| Sign/Date :
Head QC/Designee |
Sign/Date :
Head QA/ Designee |
||
Training :
| Training (If required)
|
Date :
Purpose of training: Training conducted by : Topics covered : |
|||
| Sr. No. | Name of members | Signature | Remarks of Trainees | |
| Sign/Date :
Head QC/Designee |
Sign/Date :
Head QA/Designee |
|||
Investigation Flow Chart. (Annexure -3)
Some examples of Laboratory Incident. (Annexure -4)
1.0 Laboratory Incident Type – Chromatographic issues like,
-
- Baseline disturbance, system suitability failure (e.g. tailing factor, theoretical plate, bracketing % RSD failure, resolution failure or as applicable).
-
- Error identified in the preparation of chromatographic sequence or Analytical Method during or after completion of analysis.
-
- The analyst kept the vial at an incorrect position, which is identified after the acquisition of injections in chromatographic analysis.
-
- Placebo/ blank/ impurity peak merged with any other peak of interest either in the standard or test sample.
-
- Different/atypical elution pattern in chromatographic analysis.
-
- Improper peak shape or peak splitting observed during analysis.
-
- Retention time-shifting during analysis as per SOP on General Practice of HPLC/ UPLC/GC.
-
- Area variation observed between replicate injections of test preparation and pressure drop during analysis.
-
- Chromatographic system or any analysis stops due to any error or instrument malfunctioning during analysis.
-
- Carryover of standard/ sample observed in the subsequent run (but same shall be established through hypothesis study).
-
- Correlation not established between two standard preparations as per SOP on General Practice of HPLC/ UPLC/GC.
-
-
Note:
-
-
- In all the above cases if the sample is not injected in the chromatographic system, then no need to investigate as per lab event SOP,
-
- However, it has to be justified & documented on Chromatogram/ Raw data/ Checklist and corrective actions taken to resolve the issue shall also be documented.
-
- This justification & documentation shall be reviewed by the reviewer for the adequacy before planning the new analysis.
-
- If in any case sample is injected, that has to be calculated and if results found OOS/OOT then follow the respective SOP for investigation.
-
- If results meet the specification but need to repeat then that required to investigate as per lab incident SOP with authorization
2.0 Laboratory Incident Type – Other Examples,
-
- The mobile phase is exhausted or consumed during analysis or without completion of the sample sequence.
-
- Extraneous peak investigation identified as per the SOP on Investigating and identifying extraneous peaks during chromatographic analysis.
-
- Endpoint not achieved in Karl-Fischer &Autotitrator analysis.
-
- In autosampler of dissolution apparatus, skips sampling during analysis/ wrong sample program.
-
- In Bacterial Endotoxin Test (BET), positive control is showing negative or negative control showing positive results.
-
- For Bacterial Endotoxin Test (BET) by a portable testing system, if test suitability for product positive control is more than the specified limit.
-
- For Liquid particle counter, air bubbles observed in the syringe, or syringe jammed due to viscous product and message observed “test did not fully complete”
-
- Failure to meet acceptance criteria during method validation/ transfer/ verification experiments.
-
- Spillage of any solution before subjecting to analysis/during analysis.
-
- Analyst error identified (i. e. wrong volumetric flask, wrong sample weight taken, etc.) prior to or after analysis (If the result is not OOS/OOT).
-
- % RSD failure during the standardization of volumetric solutions and Indicator sensitivity failures or indicator is not responding during analysis.
-
- Suspicious value/results, where the critical values changed by overwriting/cross out in the raw data.
-
- In the chromatographic system, if “data missing” message is observed.
Investigational tool – Fish Bone Diagram (Annexure – 5)
Guidance for Hypothesis study (Annexure – 6)
Some example of Hypothesis/ simulation study given but not limited to,
Initial laboratory Hypothesis:
- The final solution in the same vial.
- The final solution in the new vial.
- Re-dilution of stock solutions.
- Filter compatibility/ conditioning.
- Extra sonication.
- Any other.
Extended Laboratory Hypothesis:
- Sonication temperature increase/ decrease/ too much chilled.
- Uses of wrong solvent/ reagents.
- Challenging of solution stability.
- Glassware contamination.
- Any other
Target Date Extension of Incident/Investigation Report. (Annexure – 7)
Event Report No. ____________________
Initial Target Completion date : __________________
Current status of Investigation :
Pending activities for Investigation :
Reason for delay :
Justification for Delay
Revised target Date :
| Prepared by | Reviewed by (Head QC/Designee) | Approved by (Quality Head) |
|
|



Pingback: Good Laboratory Practices (GLP) - SOP & Guideline - CSTORE TRAINING
Pingback: Out of Specification (OOS) Handling Procedure - Guidelines - SOPs
Pingback: Analytical Method Transfer - Acceptance Criteria - Guidelines - SOPs
Pingback: SOP for Out of Trend (OOT) Analytical Test Results - Pharma Beginners