 Standard Operating Procedure for Laboratory Instrument and Equipment calibration through Internal or External (Third Party). This SOP includes the calibration procedure, schedule/planner and frequency of Instrument calibration in quality control laboratory.
Standard Operating Procedure for Laboratory Instrument and Equipment calibration through Internal or External (Third Party). This SOP includes the calibration procedure, schedule/planner and frequency of Instrument calibration in quality control laboratory.
SOP for Laboratory Instrument Calibration
1.0 Purpose:
-
- The purpose of this SOP ( Standard Operating Procedure) is to describe the procedure for the calibration schedule and calibration practices of Instrument/Equipment.
2.0 Scope :
-
- This SOP is applicable to the calibration of Instrument/Equipment maintained by the Quality control Department in the pharmaceutical product manufacturing plant.
3.0 References, Attachments, and Annexure:
-
-
References:
- SOP for Handling of Out of Calibration (OOC)
-
-
- In house
-
-
Attachments:
-
-
- Instrument/Equipment Master list (Attachment -1)
-
- Internal Calibration schedule (Attachment -2)
-
- External Calibration schedule (Attachment -3)
-
- Instrument/Equipment Outside Calibration Log (Attachment -4)
-
- Sieve code numbering logbook (Attachment -5)
-
- Annexure: NA
4.0 Responsibilities:
-
-
QC Officer:
-
-
- To analyze as per Calibration SOP and report the same.
-
- Handling out of calibration result.
-
-
Executive QC or designee:
-
-
- Preparation of the calibration schedule.
-
- Monitoring the calibration of Instrument/Types of equipment as per schedule.
-
- To intimate the outside agencies for the calibration of the Instrument/Types of equipment.
-
- To check the Instrument/Equipment calibration raw data and status label on Instrument/Types of equipment.
Related: SOP for Operation and Calibration of UV Cabinet
-
- Maintaining the calibration records and other respective documents.
-
- Issuance of the calibration template as per the schedule.
-
- To maintain all standards, reagents, Instrument/Types of equipment, etc. required for
-
- To verify the third party calibration report and raw data.
-
-
Quality Control Head or Designee :
-
-
- Ensure the calibration of all Instrument/Equipment performed as per schedule and calibration procedures are followed as per respective Instrument/Equipment SOP.
-
- To ensure that the calibration status is displayed over the Instrument/Equipment.
-
- To ensure all calibration documents are properly maintained.
-
-
Quality Assurance :
-
-
- Ensure the implementation of the SOP.
-
- Review and check the SOP.
-
- Regulatory Affairs, Quality Head and Plant Head or Designee:
-
- To review and approval of SOP.
-
- To ensure the implementation of the SOP.
5.0 Procedure for Instrument Calibration and Schedule Preparation:
-
-
Calibration schedule preparation and updation of Instrument/Equipment master list
- QC Executive or Designee shall prepare a two-calibration schedule as follows.
-
-
-
- Internal calibration
-
-
-
- External (Third-party) calibration
-
-
- Note: Calibration of Instrument/Equipment/device used for Calibration of QC Instrument/Equipment shall be covered under the External Calibration schedule.
Related: Maintenance of Laboratory Instrument
-
- Prepare the Instrument Calibration schedule on a yearly basis.
-
- Calibration schedule shall contain “Sr No.”, “Instrument/Equipment”, “Code No.”, “Frequency”, “Tolerance” and “Calibration due on” columns.
-
- “Calibration due on” column is subdivided as “Month” and “Sign/ date”.The calibration schedule shall contain “History” pages.
-
- “History” contains the column of “Sr. no.”, “Date”, “History details”, “Name of Instrument/Equipment”, “Code No.”, “Done by/Date” and “Approved by/Date”.
-
- Include the one more column into the above format for the External calibration schedule as “Party”. (Attachment-3)
-
- Calibration schedule shall have a provision of “Prepared by/Date” and “Verified by/Date” (Attachment-2 & 3).
-
- QC Executive or Designee shall prepare the calibration schedule and sign as “Prepared by/Date” and submit to QC Head for verification.
-
- After verification QC Head shall sign the calibration schedule as “Verified by/Date”.
-
- If any new Instrument/Equipment received in quality control department then prepare the calibration schedule for the same as addendum 01,02,03…of Attachment-2 & Attachment-3 accordingly.
-
- Maintain the Frequency and tolerance as per the respective Instrument/Equipment operation/Calibration SOP.
-
-
QC Executive or Designee shall update the “Instrument/Equipment History card ” in case of following or other cases that are not mentioned below.
-
-
-
- In case if Instrument/Equipment is not in use.
-
-
-
- Change in calibration frequency.
-
-
- For any new Instrument/Equipment, the Instrument/ Equipment handling and updation of Instrument / Equipment master list shall be as follows.
Related: SOP for Disintegration Apparatus (DT)
-
-
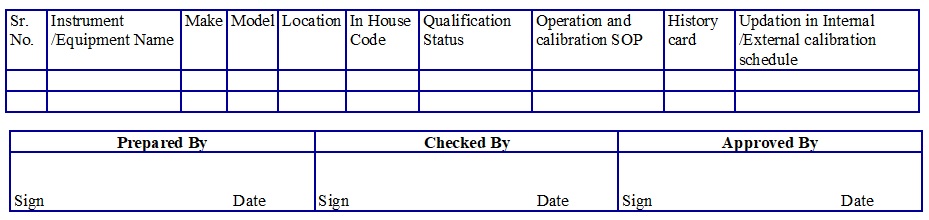
- Allot the Instrument/Equipment code no. from Instrument/Equipment Master list as follows. (Attachment-1)
-
-
-
- Instrument code no.: QXXX
-
-
-
- Where,
-
Q: Department Code (For Quality Control)
XXX: Stands for the serial no. of Instrument/Equipment.
-
-
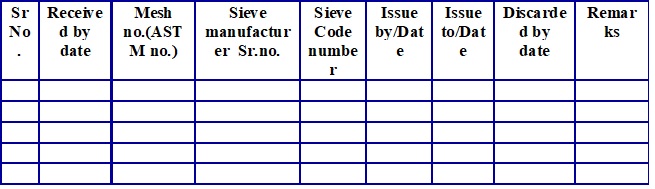
Write the code and other details of sieve in sieve code numbering logbook (Attachment-5) and allot the code number of the sieve as follows.
- Code number-SE/XXX
-
-
- SE denotes: Sieve XXX denote sieve code number.
-
- Allot the code no. of Equipment as follows: CIXX Where XX denotes Serial no. starts from 01, 02… respectively CI Denotes: Equipment and put the details in the master list separately.
-
- Perform the IQ/OQ/PQ (if required).
-
- Prepare the operation and Calibration SOP where the detail of calibration frequency shall be mentioned.
-
- To update the Calibration schedule as per Calibration SOP.
-
- If Calibration procedure and the procedure followed for OQ/PQ is similar to no additional calibration.
-
- Perform the PQ and Consider the PQ as initial calibration.
Related: Operation and Calibration of Analytical Balance
-
- In the case where the calibration procedure and the procedure followed for PQ are different additional calibration as per SOP shall be performed after PQ and before usage and this shall be considered as initial calibration.
-
- In case of any breakdown or replacement of any damaged part where calibration needs to be carried out, the calibration schedule shall not get affected.
-
- Date of Calibration shall be fixed and calibration shall be performed within the tolerance time.
-
- The next calibration due date shall remain unchanged and shall not be based on the date of the previous calibration.
-
-
Internal Calibration Practices for Laboratory Instrument :
-
-
- QC Executive or Designee shall Prepare the calibration master format as per respective SOP for all Instrument/Equipment.
-
- QC Executive or Designee shall issue the calibration format by stamping the photocopy of the master calibration format with the “WORKING COPY” stamp as per the requirement of the calibration schedule.
-
- Executive or Designee shall put the stamp of “WORKING COPY” on the photocopy of master calibration format for respective Instrument/Equipment with sign and date. For the “WORKING COPY” stamp.
Related: Raman Analyzer – Handling Procedure (SOP)
-
- In case of any breakdown or additional calibration due to replacement of any part or maintenance, QC Executive or Designee shall issue the calibration format of Instrument/Equipment.
-
- The analyst shall follow the procedure of the respective Instrument/Equipment SOP for the calibration.
-
- Submit the raw data for review to a designated person after completion of the calibration.
-
- After satisfactory calibration, the analyst shall update the status label of the calibration.
-
-
Calibration practices for the External Calibration :
-
-
- Calibrate Instrument/Equipment and equipment by the third party in the presence of a QC Executive or designee. ( when calibration performed in laboratory premises)
-
- Third-party shall follow the SOP previously submitted by them and authorized by QA or as per the approved procedure of QC.
-
- Third-party shall submit the calibration report along with raw data(where available).
-
- QC/QA Executive or Designee shall review the calibration report along with raw data and put the reviewed by stamp and signature with date.
-
- QC Executive or designee shall verify the third party calibration label on the Instrument/equipment and equipment.
-
- For the Instrument/Equipment and Equipment used for calibration, the third party shall provide the certificate with national traceability.
-
- After the completion of calibration, QC/QA Executive or Designee shall make an entry in the calibration schedule for the actual performance.
-
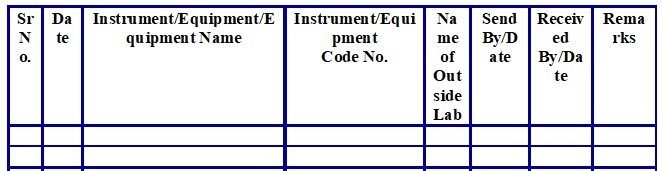
- The analyst shall send the Instrument/Equipment outside as per requirements and maintain the record as per Attachment-4.
-
-
General Procedure :
- The following shall be the tolerance criteria for the calibration of Instrument/Equipment.
-
-
- 15 days (Fortnightly) ± 2 days
-
- 30 days (Monthly) ± 3 days
-
- 60 days (Bi-monthly) ± 6 days
-
- 90 days (Quarterly) ± 7 days
-
- 180 days (6 Monthly) ± 15 days
-
- 365 days (Yearly) ± 30 days
-
- If Instrument/Equipment is not calibrated within tolerance days, due to under maintenance status,
-
- Make a note in the “History” of the calibration schedule and take the authorization of Head QC on the Calibration schedule.
Related: Good Laboratory Practices for Workbench
-
- After the review of raw data, QC Executive or Designee shall update the calibration schedule with sign and date. Consider this date as the date of calibration.
-
- If usage of Instrument/Equipment is discontinued, make a note in the “History” of the calibration schedule and mention “NA” in the column of “Sign/ date” of the calibration schedule.
-
- Made the same entry in the Instrument/Equipment history card.
6.0 Attachments for Instrument Calibration :
Instrument/Equipment Master list (Attachment -1)
Internal Calibration schedule (Attachment -2)
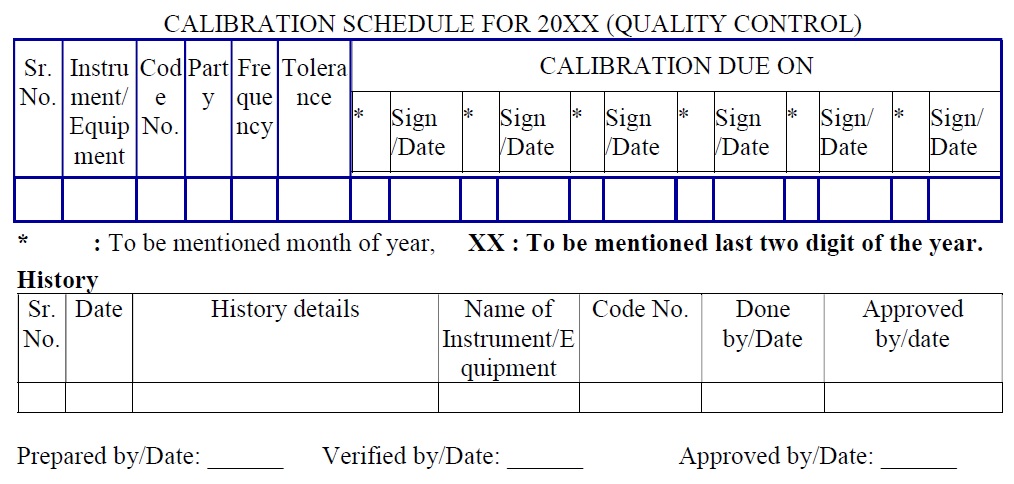
External Calibration schedule (Attachment -3)
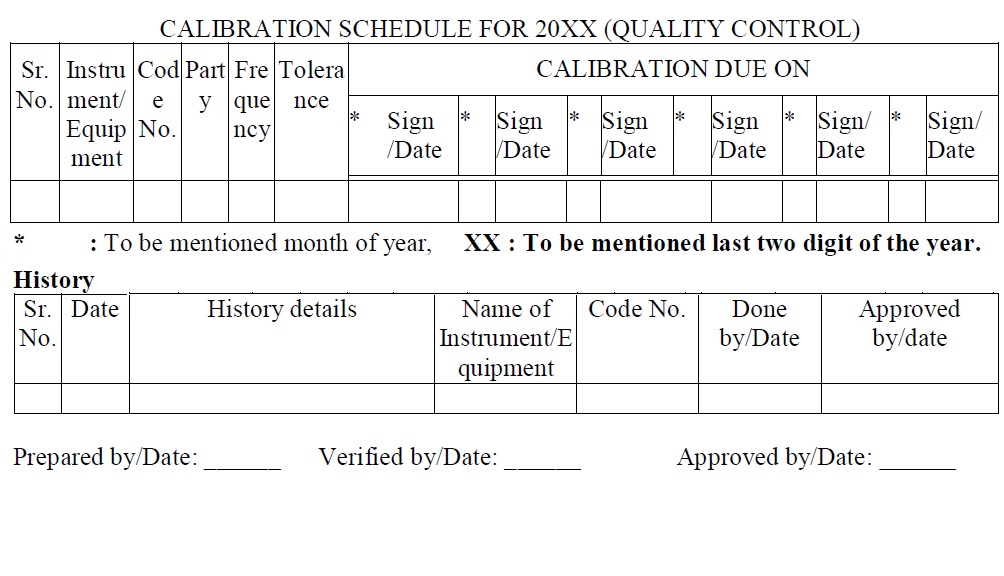
Instrument/Equipment Outside Calibration Log (Attachment -4)
Sieve code numbering logbook (Attachment -5)



Pingback: Calibration of Instrument / Equipment Policy - Guidelines - SOPs
Pingback: Laboratory Investigation Checklist - OOS Result - Guidelines - SOPs
Pingback: Stability Chamber / Incubator Management Procedure - Guidelines - SOPs
Pingback: Viscometer - Operation and Calibration Procedure - Guidelines - SOPs
Pingback: Good Laboratory Practices (GLP) - SOP & Guideline - Pharma Beginners