Standard Operating Procedure (SOP) for Review of Analytical Report and Raw Data. Analytical raw data is data generated during sample analysis and includes traceability of equipment and reagents used for analysis e.g. name of the material, instrument ID, template, chromatograms (Electronic and hard copy), the potency of reference standard / working standard /reagent, calculations, results, COA, etc.
SOP for Review of Analytical Report and Raw Data
1.0 Purpose:
-
- The Purpose of this SOP is to describe the procedure for review of the raw data of analysis carried out in the Quality Control laboratory
2.0 Scope:
-
- This SOP is applicable to Quality Control Laboratory of pharmaceutical manufacturing plant for :
-
-
- Analysis and Reporting.
-
-
-
- Review Process and Observation Reporting.
-
-
-
- Observation Compliance.
-
-
-
- ObservationsTrend Analysis.
-
3.0 References and Annexures:
-
-
References :
-
-
- Investigation of Out of SpecificationAnalysis Result (SOP)
-
- SOP For Handling of Out of Calibration.
-
- SOP for Role and responsibility of QA of QC
-
- Handling of Lab event (SOP)
4.0 Responsibilities:
-
-
Quality Control Analyst shall be responsible for :
-
-
- Perform the analysis as per the approved specification/ATP/Protocol.
-
- Report the Analytical raw data and submit for review as per the respective SOP.
-
- To give the proper cross-reference for each test in case the test results of a specific batch is filed with any other report ((i.e other Batch No. or A.R.No.) for identification and easy tracing/ tracking of data during the review.
-
- Submit an analytical reports / Raw Data after completion of analysis along with all supporting data to section head /Reviewer for review.
-
- To correct the observations given by the reviewer.
-
- To report any event/lab event, incident, deviation, OOS, OOC, OOT, etc. to the Head QC or designee.
-
-
Analytical Raw Data / Report Reviewer shall be responsible for :
-
-
- Review the analytical raw data for adequacy and accuracy, as per (but not limited to) the checklists.
-
- Provide the on-job training to the analyst as per requirements.
-
- To ensure the adequacy of compliance for the observations.
-
- Ensure handling of event/lab event, incident, deviation, OOS, OOC, OOT, etc. as per the respective SOP.
-
-
Head QC or Designee shall be responsible for :
-
-
- To ensure review of raw data of analysis as per the SOP
-
- To ensure adequate compliance/justification for the observations.
-
- Evaluate training needs based on the observation and to ensure training is given to the analyst, wherever required.
-
- To ensure handling of event/lab event, incident, deviation, OOS, OOC, OOT, etc. as per the respective SOP.
-
- To ensure that analysis is carried out as per authorized document and reported inappropriate analytical template/worksheet.
-
-
The technical person from Quality Assurance department shall be responsible for :
-
-
- To check the SOP.
-
- Ensure the implementation of the system as per the SOP.
-
- Review the paper and electronic raw data and records generated in the QC laboratory as a secondary check by QA.
-
-
Regulatory Affairs, Quality Head and Plant Head shall be responsible for :
-
-
- To review and approve the SOP.
5.0 Abbreviations and Definition of Terms :
-
- Abbreviations :
- ATP : Analytical Test Procedure.
- AR No. : Analytical Report Number.
- API : Active Pharmaceutical Ingredient.
- BET : Bacterial Endotoxin Test.
- CSE : Control Standard Endotoxin
- CFU : Colony Forming Unit.
- DHS : Dry Heat Sterilization.
- GSM : Gram per Square Meter
- GMP : Good Manufacturing Practices.
- IC : Ion Chromatography.
- IR : Infra-Red.
- LOD : Loss On Drying
- LRW :Lal Reagent Water
- MLT : Microbial Limit Test.
- MVD : Maximum Valid Dilution
- NIST : National Institute of Standards and Technology
- OOS : Out of Specification.
- OOT : Out of Trend.
- OOC : Out of Calibration.
- PQ : Performance Qualification
- RS : Reference Standard.
- RSD : Relative Standard Deviation.
- RPM : Revolution Per Minute
- TOC : Total Organic Carbon
- WS : Working Standard
6.0 Procedure – Review of Analytical Raw data and Report :
-
-
Analysis and Reporting:
-
-
- Analyst shall use the approved/issued, template/ handbook /Protocol/worksheet for raw data recording.
-
- Analyst shall perform the analysis as per approved analytical test procedure or protocol.
-
- Before starting the analysis, analyst shall verify Calibration status of instrument(s) to be used, sufficient quantity of volumetric solutions/reagents, column required for the analysis, Current version of Specification, test procedure, protocol and template/worksheet issued for analysis, etc.(but not limited to).
-
- All the solutions used in the analysis shall be properly labeled and retained in respective storage conditions until the results of the analysis are derived.
-
- For microbiological analysis, solutions may be stored or may not be stored depending on the need for the investigation.
-
- Fill all records by an indelible ballpoint pen for long term legibility. (Use of pencil is not allowed except for TLC analysis).
-
- Make all entries online in legible handwriting and signed with the date.
-
- Record the date and time format as per the SOP of Documentation Practices.
-
-
Any unauthorized paper or pieces of paper shall not be used for data recording.
-
-
- The analyst shall make necessary entries online in log books for instruments/ columns/ reference standard usages and for the reagent/ solution/ buffer/ indicator preparations/ standardization and put sign & date wherever applicable.
-
- Example: Instrument ID, Expiration date, batch No./ potency and Expiration date of reference standard/ impurity standard /working standard, make/ grade and validity of reagent’s / buffer/ indicator solution, etc.
-
- The analyst shall make corrections by striking out of the original value with a single line and make the correct entry beside that and put sign and date (write the justification for incorrect entry if required) (Corrective fluid or erasable pens shall not be used to correct the original information).
-
- The analyst shall strikeout a page or section of the page which is not used, with a single diagonal line, enters “NA” and put the signature with date.
-
- After completion of the analysis, the analyst shall compile the chromatogram / spectrum / histogram / TLC plate, etc. and affix with the template /worksheet/ analytical report.
-
- The analyst shall attach a consolidated summary page for each report (i.e data packet) in order to reference where the test results for a specific lot of the product/ material are filed.
-
- When more than one batch/ A.R.No. are run together in the same sequence on the system, then the analyst shall compile the report by attaching the complete run sequence, along with all the standard chromatograms and the sample chromatograms of the particular batch along with other required details.
-
- The analyst shall write the cross-reference for each required test( e.g appearance, dissolution, CU, Assay, RS, etc.)
-
- If the data is filed with a different report (i.e Batch No./A.R.No.) so that identification, tracing/tracking of the data becomes easy during the review.
-
-
The analyst shall ensure raw data and calculations are accurate and complete.
-
-
- The analyst shall report any event/lab event, incidence, deviation, OOS, OOC,OOT, etc. to the Head QC or designee, as soon as it occurs as per respective SOP for further handling.
-
- All analytical calculations shall be performed through software like LIMS, Empower, etc.
-
- Excel spreadsheets must be qualified and validated for accuracy & reliability (wherever the excel sheet is used for calculations.)
-
- Manual calculations shall be reported if the software-based calculation option for the test is not available or in the case when multiple calculations of the same test need to be reported.
-
- All the Handwritten or manual calculations performed by the analysts shall be verified for correctness by the reviewer during the data review.
-
-
Review Process and Observation Reporting :
-
-
- Raw data of any analysis shall be reviewed within two working days from completion of the analysis.
-
- After verification of raw data /analytical reports, the analyst shall submit the analytical report to the reviewer with all supporting data for review.
-
- The reviewer shall review all the tests/ results against current practices, SOP, specification and ATP.
-
- Verify correct reporting of raw data, calculations and cross-references and details (Batch No./ potency and expiration dates of reference standard/ impurity standard /working standard, make/ grade and validity of reagent’s / buffer’s/ indicator solution, instrument calibration expiration dates, etc.) given in raw data/ hard book of respective tests.
-
- Ensure the proper traceability of documents, analyst’s signature, and results reporting, cancellation of blank space in raw data/ hard book with proper justification and also check for any relevant additional attachments attached.
-
- Reviewer shall check the entries in the logbooks of instrument and equipment used for analysis and shall verify the calibration/qualification status at random and it should be in chronological order of activities.
-
- Use only Ballpoint pen for signing the logbooks, checking the analytical raw data, analytical worksheets, and issuance of Annexures and Attachments of the SOP.
-
- The reviewer shall ensure that the results are correctly reported as per Rounding off figures as per SOP for Rounding Off the Analytical Test Results.
-
- The reviewer shall check all the individual tests and parameter as per checklist, but not limited to, the “checklists for raw data review” as per Annexure No.1 to 10.
-
- Verify the electronic data during the raw data review. The hard copy should be in line with electronic data. The reviewer shall also review the audit trail details.
-
-
Reviewer shall examine chromatographic data through the software on a periodic basis as per the following content ( but not limited to.)
-
-
-
- Single injections.
-
-
-
- Unprocessed chromatograph.
-
-
-
- Discontinued sequences.
-
-
- The reviewer shall put checked by signature and date after completions of checking of the raw data for each test.
-
- Ensure that, any event/ lab event, incident, OOS, OOC, OOT and deviation are investigated as per the respective SOP.
-
- The reviewer shall ensure that all the mentioned justifications are logical, scientifically proofed and traceable.
-
- The reviewer shall ensure the closed status of event/lab event, incident, OOS, OOC, OOT and deviation encountered during analysis.
-
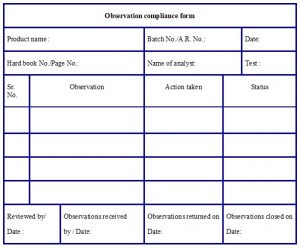
- Any discrepancy observed by the reviewer shall be reported to the Head QC or designee through the “Observation compliance form” (refer Annexure – 11).
-
-
The reviewer shall discuss the discrepancies with analysts and if required, provide training to the analyst.
-
-
- The reviewer shall retain the copy and maintain the file of the issued observation compliance form.
-
- Further, all GMP impacted document like Usage logs of Instrument, column, reference standard, working standards, material inward registers (Raw Material/Packing Material/In-process/Intermediate, etc.), etc. are to be verified on periodic basis by Section Head/designee/Reviewer for any incomplete entries, blank entries, etc. and shall put the signature& date after review at the bottom of the page.
-
- Perform the activity on a monthly basis for such periodic review or as and when required.
-
-
Observation Compliance :
- Head QC or designee shall ensure compliance of observations and provide recommendations for corrective/ preventive action.
-
-
- The analyst shall provide details of correction in the “Action taken” column after discussion with section head.
-
- Analyst or Section head shall fill the issued observation compliance form and returned to the reviewer.
-
- The reviewer shall ensure the correction takes place and submit the document for the approval to Head QC.
-
-
Observation Trend Analysis :
- Head QC or designee and QA of QC shall assess the observations made by reviewers on a quarterly basis and tabulate the repeat observations. Repeat observations are defined as the same error or event in a given category.
-
-
- If the same observation is more than three times quarterly, Consider as repeat observation.
-
- Head QC or designee and QA of QC shall review the data and evaluate the repeat observations against analyst as per following but not limited to,
-
-
- Entries / Signature missing.
-
-
-
- Overwriting / Incorrect entries.
-
-
-
- Incorrect calculation/ Preparation /ATP not followed.
-
-
-
- Justification not written.
-
-
-
- Incorrect material analyzed
-
-
- The reviewer or designee shall prepare the graph (Bar type) for the type of observations (X-axis) against No. of observations for the analyst (Y-axis).
-
- Reviewer or designee shall evaluate the repetitive type of failures for all analysts,
-
- The reviewer or designee shall also prepare a graph (Bar type) for the number of observations for each analyst (X-axis) against total No. of observations made by all analysts in the quarter (Y-axis).
-
- The reviewer or designee shall use the data of repeat observations for the identification of training needs. Changes and/ or clarification in the method of analysis etc. may be made as results of trend analysis.
-
- Head QC shall ensure the completion of preventive action.
7.0 Annexures – Review of Analytical Raw data and Report:
-
-
Annexure – 1: Checklist for review of raw material/ in-process / finished Product/ stability sample analysis /Other analysis.
-
-
- To download /view the Checklist, Click here
-
-
Annexure – 2:Checklist for review of packing materials analysis data.
-
|
Sr. No. |
Test |
Checking Parameter |
|
1. |
Product information and general information as per Annexure-1 | |
|
2. |
Chemical test /IR / UV test |
|
|
3. |
Test as per respective specification and ATP |
|
-
-
Annexure– 3: Checklist for review of microbiology analysis data.
-
-
- To download /view the Checklist, Click here
-
-
Annexure– 4: Checklist for review of outside laboratory testing report.
-
Checkpoints – Review of Analytical Raw data and Report
-
- Name of the sample against test request.
-
- Batch number.
-
- Method of analysis followed.
-
- Reference number of the sample analyzed (pharmacopeia or in-house).
-
- Reported result as per the specification.
-
- Chromatograms along with the report (if applicable).
-
- Signature of analyzed by and authorized by.
-
- Review of raw data and COA.
-
- Put the stamp of reviewed/verified by initial and date.
-
-
Annexure – 5: Checklist for review of instrument calibration data.
-
Checkpoints – Review of Analytical Raw data and Report
-
- Name of the instrument.
-
- Name and traceability certificate of master device /instruments which have been used for calibration.
-
- Calibration status of master devices /instruments used for calibration.
-
- Operating range Vs calibration range.
-
- Date of calibration and next calibration due date of the instrument.
-
- Any deviation from the due date.
-
- Calibration as per respective SOP/template/worksheet.
-
- Result as per the specification.
-
- Analyst signature.
-
-
Annexure – 6: Checklist for review of the outside laboratory calibration report.
-
Checkpoints – Review of Analytical Raw data and Report
-
- Name of the instrument.
-
- Code number or reference number of instruments.
-
- Date of qualification or calibration.
-
- Review of certificate, acceptance criteria, valid up to date.
-
- Operating range Vs calibration range.
-
- The accuracy of the master instrument used for calibration should be stringent than the instrument to be calibrated.
-
- Calibration status of instruments used for calibration of our equipment.
-
- Acceptance criteria.
-
- Put the stamp of reviewed/verified by initial and date.
-
-
Annexure – 7: Checklist for review of buffer, reagent, indicator volumetric solution preparation and standardization data.
-
Checkpoints – Review of Analytical Raw data and Report
-
- The primary standard used should be traceable to NIST/ Pharmacopoeial grade.
-
- Certificate of primary standard and expiration date.
-
- Drying of the primary standard if applicable before use.
-
- Storage of solution prepared for analysis.
-
- The entry of primary standards used in respective logbooks.
-
- Grade of a reagent which is used in different solution preparation.
-
- The physical appearance of the solution which has been prepared.
-
- Date of preparation, solution expiration date.
-
- Strength of solution.
-
- Pharmacopoeial status.
-
- The normality of a volumetric solution must be within the limit.
-
- Prepared by / date.
-
- Calculation & result must meet the specification limit.
-
-
Annexure – 8: Checklist for review of working standard qualification data
-
Checkpoints – Review of Analytical Raw data and Report
-
- Name of working standard.
-
- Pharmacopoeial status.
-
- Date of preparation and valid up to date.
-
- Analysis against a current lot of reference standards.
-
- Certificate/potency of the reference standard and expiration date.
-
- Characterization data.
-
- The analysis was done as per the respective SOP.
-
- Parameters of the test as per Annexure-1 which are applicable.
-
-
Annexure – 9: Checklist for review of method validation/method transfer data
-
Checkpoints – Review of Analytical Raw data and Report
-
- Product information and general information whatever applicable as per checklist-1.
-
- The method as per given reference specification /ATP.
-
- Acceptance criteria.
-
- Strategy design.
-
- Placebo preparation.
-
- Extra peaks observed.
-
- Acceptance criteria described in the validation protocol (Complies on Not Complies).
-
- Any method limitation is given in the validation report.
-
- Any deviation from protocol and justification.
-
- Final conclusion and recommendation.
-
-
Annexure – 10:Check list for review of instrument/ equipment qualification data
-
Checkpoints – Review of Analytical Raw data and Report
-
- Purchase order copy.
-
- Operation and maintenance manual.
-
- Calibration standard used for qualification and its traceability certificate with an expiration date.
-
- Signature and date of qualification performed by and of the service engineer.
-
- Training record of the performer of PQ.
-
- Certificates availability.
-
- Certificate for the material of construction.
-
- Spares list and verification of serial no. of part of instruments/ equipment.
-
- Instrument calibration data.
-
- Results of qualification testing.
-
- Acceptance criteria of qualification protocol or requirements met.
-
-
Annexure – 11: Format for Observation compliance form
-



Pingback: Quality Assurance in Laboratory (Lab QA) - Guideline - Pharma Beginners